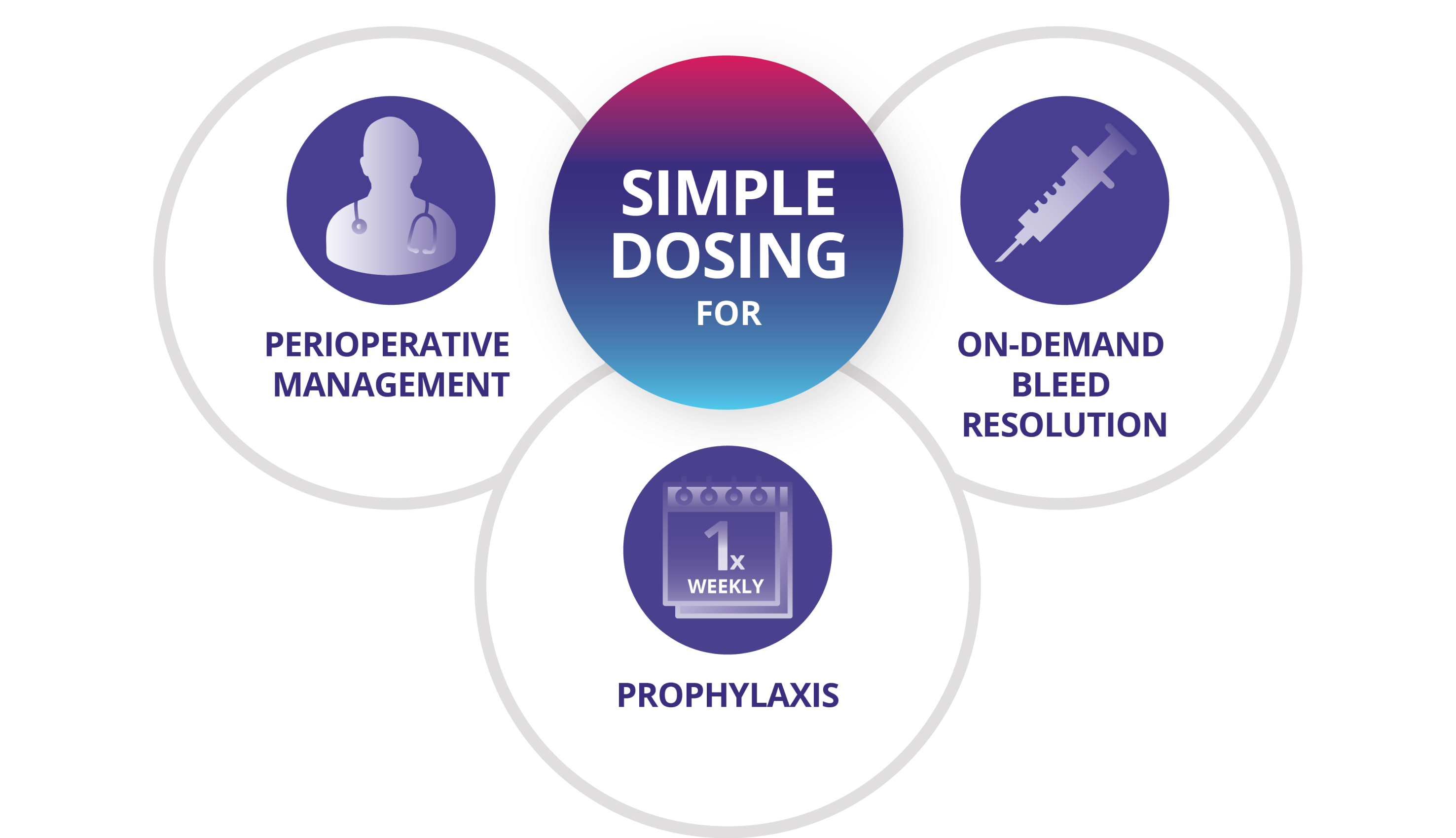
Simple dosing for all your patients' needs
Recommended dose 50 IU/kg1
Perform infusion slowly over 1 to 10 minutes, based on the patient's comfort level.
Prophylaxis
- For routine prophylaxis in adults, adolescents, and children, 50 IU/kg administered once weekly
On-Demand Bleed Resolution
- For on-demand bleed resolution, a single dose of 50 IU/kg
- For minor/moderate bleeding episodes within 2 to 3 days after prophylactic dose, 30 IU/kg dose may be used
- For minor/moderate/major bleeding episodes, additional doses of 30 IU/kg or 50 IU/kg every 2 to 3 days may be considered
Perioperative Management
- For perioperative management, a single dose of 50 IU/kg
- For minor surgery, an additional postoperative dose of 30 IU/kg or 50 IU/kg after 2 to 3 days may be considered
- For major surgery, additional postoperative doses of 30 IU/kg or 50 IU/kg every 2 to 3 days may be administered as clinically needed
Select storage requirements1
Store ALTUVIIIO in powder form at 2°C to 8°C (36°F to 46°F). Do not freeze to avoid damage to the prefilled diluent syringe.
ALTUVIIIO may be stored at room temperature, not to exceed 30°C (86°F), for a single period of up to 6 months, within the expiration date printed on the label. After storage at room temperature, do not return the product to the refrigerator.
Store ALTUVIIIO in the original package to protect vials from light.
Discard ALTUVIIIO if it is not used within 3 hours of reconstitution, if solution is cloudy or has particulate matter, or any drug is unused.
For additional information on storage, please see Section 16 of the Prescribing Information.
Dosage strengths1
ALTUVIIIO is available in 6 vial strengths to optimize administration.
These include single-dose vials containing nominally 250, 500, 1000, 2000, 3000, or 4000 international units (IU) per vial.
There’s no need for routine pharmacokinetic testing with ALTUVIIIO1
Sanofi has contracted with LabCorp (Esoterix) to provide a central lab to perform standardized, one-stage clotting assays with a national network of local laboratories for specimen collection at no extra cost to the patient or healthcare provider.
For more information about monitoring with LabCorp, contact your ALTUVIIIO Representative.
Watch a step-by-step infusion-demonstration video for your patients

Indication
IU=international unit.
Reference: 1. ALTUVIIIO Prescribing Information. Bioverativ Therapeutics Inc. Waltham, MA.