Factor VIII Activity Levels Matter
Lower Factor VIII levels put patients at greater risk for bleeds1,2
WFH Guidelines,* along with a retrospective analysis,† have shown that at Factor VIII levels of 5% to <40% (mild hemophilia), patients are vulnerable to traumatic, joint, and surgical bleeds, including subclinical bleeds.1,2
Higher Factor VIII levels are associated with the ability to perform activities with lower risk of a bleed1,3-8*‡§||
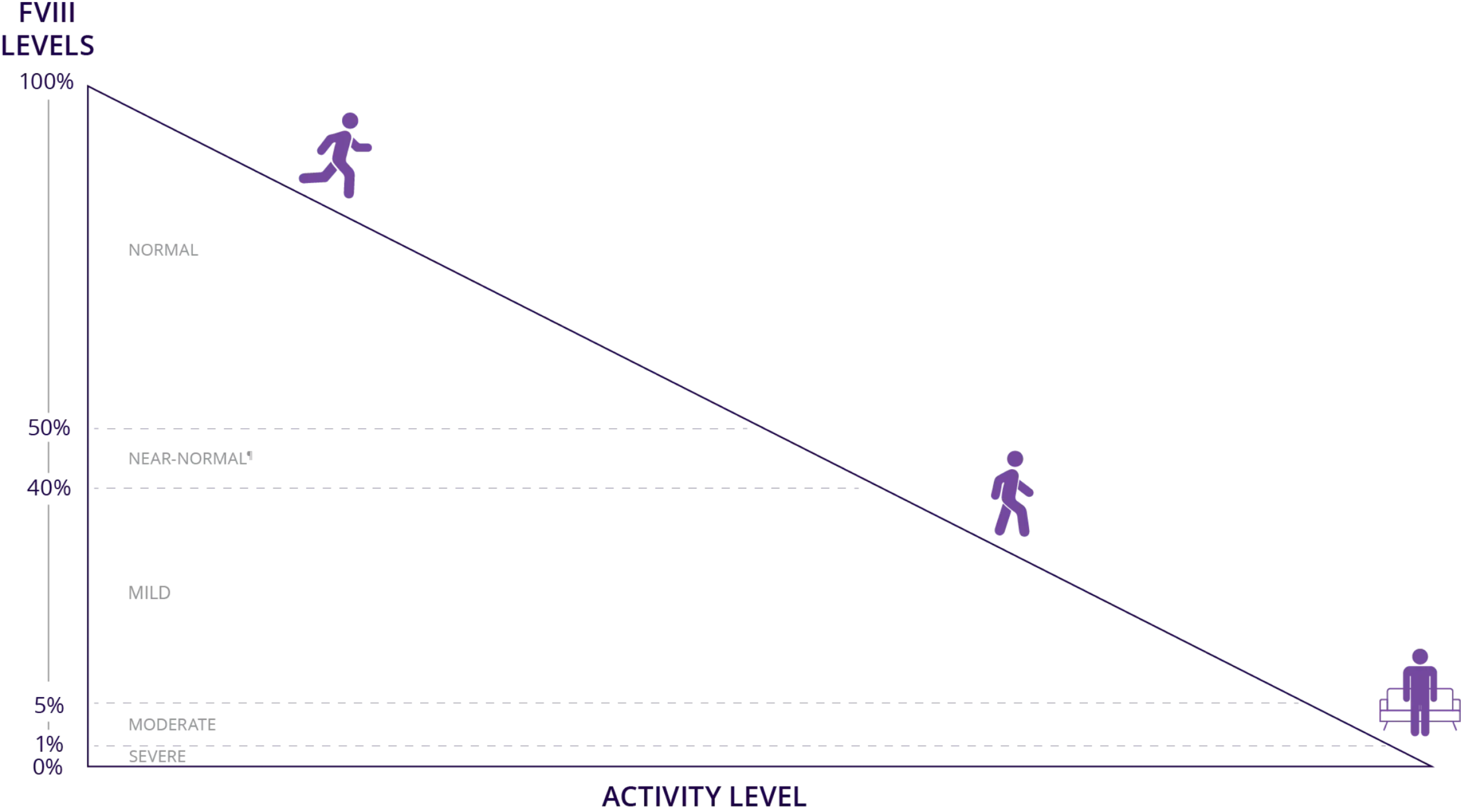
Higher Factor VIII levels reduce bleeding risk and may enable patients to live a more
active life1,3,6-8*‡§ll
* Data from the 2020 WFH Guidelines for the Management of Hemophilia, 3rd edition.1,4
† Data from a study examining a 2001 series of self-reported Dutch surveys that included 433 patients with mild or moderate hemophilia A who were treated on demand.9
‡ Data from a 2021 systematic review of physical activity in people with hemophilia across multiple databases, extracted using a standardized template and reported using a narrative synthesis.6
§ Data from an expert elicitation study using Sheffield Elicitation Framework methodology quantified with probability analysis.7
ll Data from a Delphi consensus statement using structured expert opinion to define target factor levels for use in different clinical situations.8
¶ WFH Guidelines define the upper limit of mild hemophilia as 40% factor activity and the WFH Introduction to Hemophilia defines the normal range as 50% to 150%, which indicates that 40% to 50% would be in-between mild hemophilia and normal, here referred to as “near-normal” levels.1,4,5
Is it time for a higher level of protection?
Achieving higher Factor VIII levels has the potential to:
Provide greater protection from bleeds10*
Improve physical function1,11†‡
Reduce pain1,11†‡
Preserve joint health11,12†§
The level of bleed protection depends on the levels of Factor VIII1,2‡ǁ
ARE YOUR PATIENTS REALLY OK?
71% of patients with hemophilia A say they are fine, but more than half rate their health as fair or poor.
What’s more, patients on standard or extended half-life prophylaxis still report a median of 2.7 bleeds a year.13,14¶
Which of your patients could benefit from a higher level of bleed protection?
* Data from a post hoc analysis of the association of Factor VIII levels and AUC with ABR in 34 patients on PK-guided prophylaxis.10
† Data from a randomized, controlled trial of secondary/tertiary prophylaxis vs on-demand treatment in adult and adolescent patients with severe hemophilia A over 3 years.11
‡ Data from the 2020 WFH Guidelines for the Management of Hemophilia, 3rd edition.1
§ Data from a retrospective analysis of effect of hemophilia type and factor level on joint bleeding and orthopedic procedures in 4771 males with nonsevere hemophilia A or B without inhibitors receiving on-demand factor replacement therapy over a 12-year period.12
|| Data from a study examining a 2001 series of self-reported Dutch surveys that included 433 patients with mild or moderate hemophilia A who were treated on demand.9
¶ In a global, noninterventional study that prospectively collected real-world data of 49 participants with severe hemophilia A treated with prophylactic FVIII, the mean (95% confidence interval) and median (Q1, Q3) ABRs were 5.0 (3.3, 7.5) and 1.9 (0.0, 0.82) for treated bleeds and 6.2 (4.2, 9.2) and 2.7 (0.0, 9.4) for all bleeds.14
ABR=annualized bleed rate.
Improving bleed protection may allow closer-to-normal activity for patients with hemophilia A1
Factor VIII levels and their impact on the ability to perform activities
| Factor VIII Activity Levels1,3,4,8,15 | Impact on Physical Activity1,3,8,15 |
| Normal levels 50%-150% factor activity | None to minimal High-impact activity possible with no pain (sports, physically taxing jobs). |
| Near normal* ≥40%-<50% factor activity | Near-normal factor levels are currently undefined by the World Federation of Hemophilia. |
| Mild hemophilia 5%-<40% factor activity | Minor High-risk activity is possible. Intensive sport activity may be considered for those with factor levels between 15%-30%. Appropriate level of physical activity should be evaluated on a case-by-case basis. May experience pain and limited mobility. |
| Moderate hemophilia 1%-5% factor activity | Moderate Factor levels between 1%-3% may be sufficient for those with a sedentary lifestyle, but higher levels between 3%-5% are recommended if engaging in mild physical activity. Individuals should refrain from high-risk activity. May experience pain and limited mobility. |
| Severe hemophilia <1% factor activity | Major High risk of spontaneous bleeds—even with low activity there will be pain in target joints. |
As Factor VIII levels approach normal, patients are less likely to experience bleeds1†
* WFH Guidelines define the upper limit of mild hemophilia as 40% factor activity, and the WFH Introduction to Hemophilia defines the normal range as 50% to 150%, which indicates that 40% to 50% would be in between mild hemophilia and normal, here referred to as “near-normal” levels.1,4,5
† Factor levels should be assessed based on an individual patient basis.
Indication
AUC=area under the curve; FVIII=Factor VIII; MOE=mechanism of extension; PK=pharmacokinetics; Q1=25th percentile; Q3=75th percentile; WFH=World Federation of Hemophilia.
References: 1. Srivastava A, et al. Haemophilia. 2020;26(suppl 6):1-158. 2. Chowdary P, et al. Thromb Haemost. 2020;120(5):728-736. 3. Centers for Disease Control and Prevention. Diagnosis of Hemophilia. Updated May 15, 2024. Accessed April 11, 2025. https://www.cdc.gov/hemophilia/testing/index.html 4. National Hemophilia Foundation. Hemophilia A. Accessed May 13, 2024. https://www.hemophilia.org/bleeding-disorders-a-z/types/hemophilia-a 5. World Federation of Hemophilia. Introduction to hemophilia. Updated May 2012. Accessed May 13, 2024. https://elearning.wfh.org/elearning-centres/hemophilia/_gl=1*rmb6pc*_ga*Mjg2ODM5NTI4LjE3MTU2NTgxMTM.*_ga_7974KH9LH5*MTcxNTY1ODExMy4xLjEuMTcxNTY1ODExNi4wLjAuMA.. &_ga=2.257666123.1723173588.1715658114-286839528.1715658113. 6. Kennedy M, et al. Haemophilia. 2021;27(4):544-562. 7. Martin AP, et al. Haemophilia. 2020;26(4):711-717. 8. lorio A, et al. Haemophilia. 2017;23(3):e170-e179. 9. den Uijl IE, et al. Haemophilia. 2011;17(1):41-44. 10. Valentino LA, et al. Haemophilia. 2016;22(4):514-520. 11. Manco-Johnson MJ, et al. J Thromb Haemost. 2017;15(11):2115-2124. 12. Soucie JM, et al. Blood Adv. 2018;2(16):2136-2144. 13. Data on file, September 2023. 14. Kenet G, et al. JCM. 2021;10(24):5959. 15. Skinner MW, et al. Haemophilia. 2019;26(1):17-24.