Achieving high sustained mean Factor VIII levels (>40%) for most of the week in adults by overcoming the half-life limitations of von Willebrand Factor (vWF)1-3
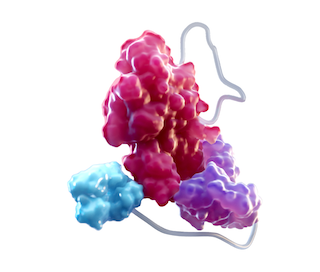
THE HALF-LIFE OF FACTOR VIII IS EXTENDED WITH NATURAL AMINO ACIDS1-4*
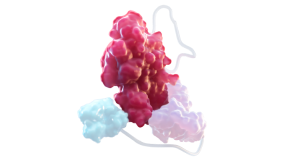
FACTOR VIII1,3,4
At the core of ALTUVIIIO is rFVIII, which replaces deficient FVIII in patients with hemophilia A
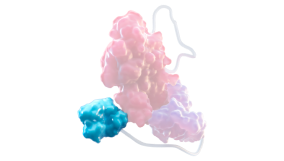
Fc DOMAIN1,3,4
Delays FVIII degradation, and helps FVIII stay in circulation by slowing the clearance rate
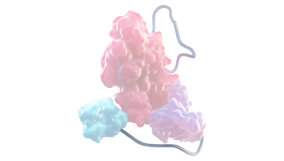
XTEN POLYPEPTIDE INSERTION1,3,4
Shields the FVIII molecule from proteolytic degradation and reduces binding to clearance receptors
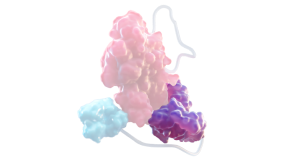
vWF D’D3 DOMAIN4
Prevents binding of FVIII to endogenous vWF
ALTUVIIIO is uniquely designed to be independent of the vWF chaperone3,4
* ALTUVIIIO is composed of naturally occurring amino acids that are able to be broken down through regular protein degradation processes.4
Discover how ALTUVIIIO achieves higher sustained mean Factor VIII levels (>40%)1-3
In a Phase 3 study, ALTUVIIIO offered a 48.2-hour half life in adults—the longest of any Factor VIII replacement therapy4-15†
† Comparison of data from XTEND-1 with half-life data recorded in prescribing information of approved Factor VIII replacement therapies.
Indication
AUC=area under the curve; FVIII=Factor VIII; IU=international unit; IV=intravenous; PK=pharmacokinetic; rFVIII=recombinant Factor VIII; SD=standard deviation.
References: 1. von Drygalski A, et al. N Engl J Med. 2023;388(4):310-318. 2. Chhabra ES, et al. Blood. 2020;135(17):1484-1496. 3. Konkle BA, et al. N Engl J Med. 2020;383(11):1018-1027. 4. ALTUVIIIO Prescribing Information. Bioverativ Therapeutics Inc. Waltham, MA. 5. Adynovate Prescribing Information. Takeda Pharmaceuticals U.S.A., Inc. Lexington, MA. 6. Afstyla Prescribing Information. CSL Behring LLC. Kankakee, IL. 7. Eloctate Prescribing Information. Bioverativ Therapeutics Inc. Waltham, MA. 8. Esperoct Prescribing Information. Novo Nordisk Inc. Plainsboro, NJ. 9. Kovaltry Prescribing Information. Bayer HealthCare LLC. Whippany, NJ. 10. Novoeight Prescribing Information. Novo Nordisk Inc. Plainsboro, NJ. 11. Nuwiq Prescribing Information. Octapharma USA, Inc. Paramus, NJ. 12. Xyntha Prescribing Information. Wyeth Pharmaceuticals LLC. Philadelphia, PA. 13. Advate Prescribing Information. Takeda Pharmaceuticals U.S.A., Inc. Lexington, MA. 14. Jivi Prescribing Information. Bayer Healthcare LLC. Whippany, NJ. 15. Recombinate Prescribing Information. Takeda Pharmaceuticals U.S.A., Inc. Lexington, MA.