First-in-class ALTUVIIIO: XTEND-1 clinical trial1
In previously treated patients (PTPs) who had received Factor VIII replacements2*
XTEND-1 Pivotal Trial2
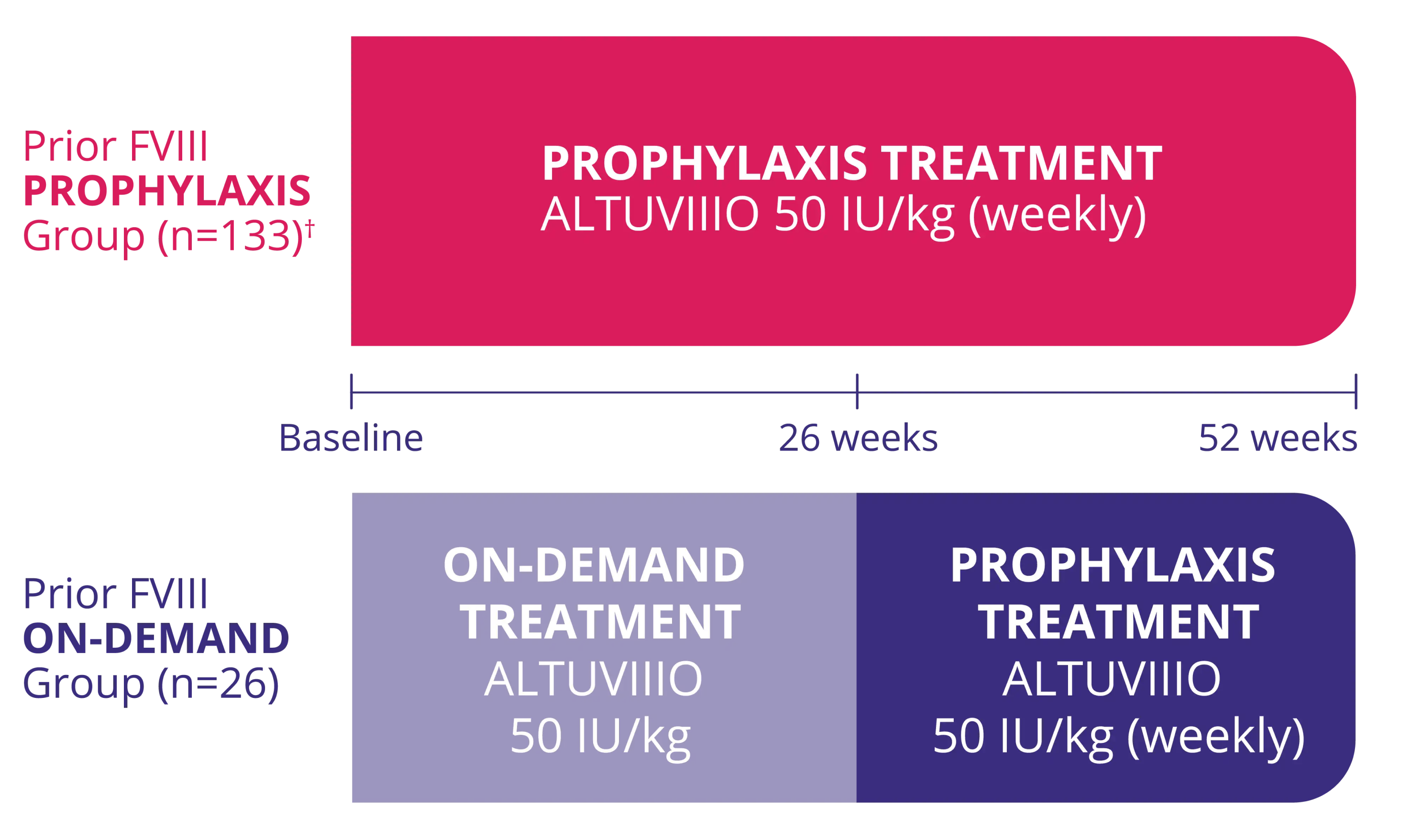
The Prior Factor VIII Prophylaxis Group included the first female patient with severe hemophilia A in a pivotal Factor VIII clinical trial1-15‡
XTEND-1 Study Design2:
A multicenter, open-label, nonrandomized trial in adults and adolescents ≥12 years (158 males and 1 female) with severe hemophilia A, including an intrapatient comparison in patients who had participated in a prospective observational study prior to enrollment in XTEND-1 study.
* Previous treatment for hemophilia A (prophylaxis or on-demand) with any recombinant and/or plasma-derived Factor VIII or cryoprecipitate for at least 150 exposure days for patients ≥12 years.16
† Efficacy of prophylaxis was evaluated in patients with at least 26 weeks of exposure to ALTUVIIIO (N=128).2
‡ Pivotal trials for Recombinate™ (Antihemophilic Factor) did not present demographic data based on sex.12
XTEND-1 Endpoints1,16
Primary Endpoint:
Mean annualized bleed rate (ABR)
Secondary Endpoints:
Intrapatient ABR comparison (key secondary endpoint)
Perioperative management
Treatment of bleeds
Quality of life (physical health and pain)
Joint health outcomes
Pharmacokinetics
Safety and tolerability
XTEND-1: A large group of 159 diverse patients were studied1
Diverse patient population (including 1 female participant)
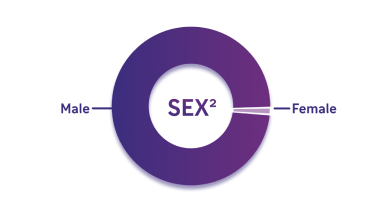
- 99% Male
- ~1% Female
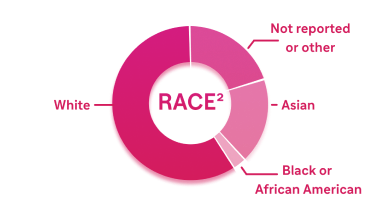
- 61% White
- 19% Not reported or other
- 18% Asian
- 2% Black or African American
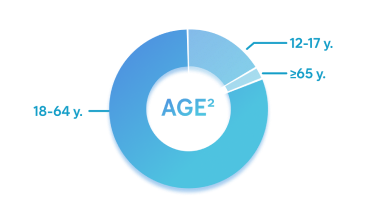
- 81% 18-64 years
- 16% 12-17 years
- 3% ≥65 years
Indication
IU=international unit.
References: 1. von Drygalski A, et al. N Engl J Med. 2023;388(4):310-318. 2. ALTUVIIIO Prescribing Information. Bioverativ Therapeutics Inc. Waltham, MA. 3. Advate Prescribing Information. Takeda Pharmaceuticals U.S.A., Inc. Lexington, MA. 4. Adynovate Prescribing Information. Takeda Pharmaceuticals U.S.A., Inc. Lexington, MA. 5. Afstyla Prescribing Information. CSL Behring LLC. Kankakee, IL. 6. Eloctate Prescribing Information. Bioverativ Therapeutics Inc. Waltham, MA. 7. Esperoct Prescribing Information. Novo Nordisk Inc. Plainsboro, NJ. 8. Jivi Prescribing Information. Bayer HealthCare LLC. Whippany, NJ. 9. Kovaltry Prescribing Information. Bayer HealthCare LLC. Whippany, NJ. 10. Novoeight Prescribing Information. Novo Nordisk Inc. Plainsboro, NJ. 11. Nuwiq Prescribing Information. Octapharma USA, Inc. Paramus, NJ. 12. Recombinate Prescribing Information. Takeda Pharmaceuticals U.S.A., Inc. Lexington, MA. 13. Clinicaltrials.gov. NCT00543439 (Xyntha prophylaxis). Sexes Eligible for Study. Accessed May 15, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT00543439 14. Clinicaltrials.gov. NCT00141843 (Xyntha bioequiv). Sexes Eligible for Study. Accessed May 15, 2024. https://clinicaltrials.gov/ct2/show/NCT00141843 15. Clinicaltrials.gov. NCT00243659 (Xyntha surgery). Sexes Eligible for Study. Accessed May 15, 2024. https://clinicaltrials.gov/ct2/show/NCT00243659 16. von Drygalski A, et al. Supplementary Appendix. N Engl J Med. 2023;388(4):310-318.