Powerful bleed protection with a once-weekly infusion1
Low ABRs in the Prior Factor VIII Prophylaxis Group1,2
ABR* with ALTUVIIIO prophylaxis (N=128)

0.0 Median ABR
(Q1, Q3: 0.0, 1.0)

PRIMARY ENDPOINT
0.7 Mean ABR†
(95% Cl: 0.5-1.0)
0.7 Mean ABR†
(95% Cl: 0.5-1.0)
* Based on treated bleeds.2
† Based on negative binomial model.2
Patients who switched to ALTUVIIIO prophylaxis experienced significant reductions in ABR2
Intrapatient ABR comparison (n=78) vs prior Factor VIII prophylaxis
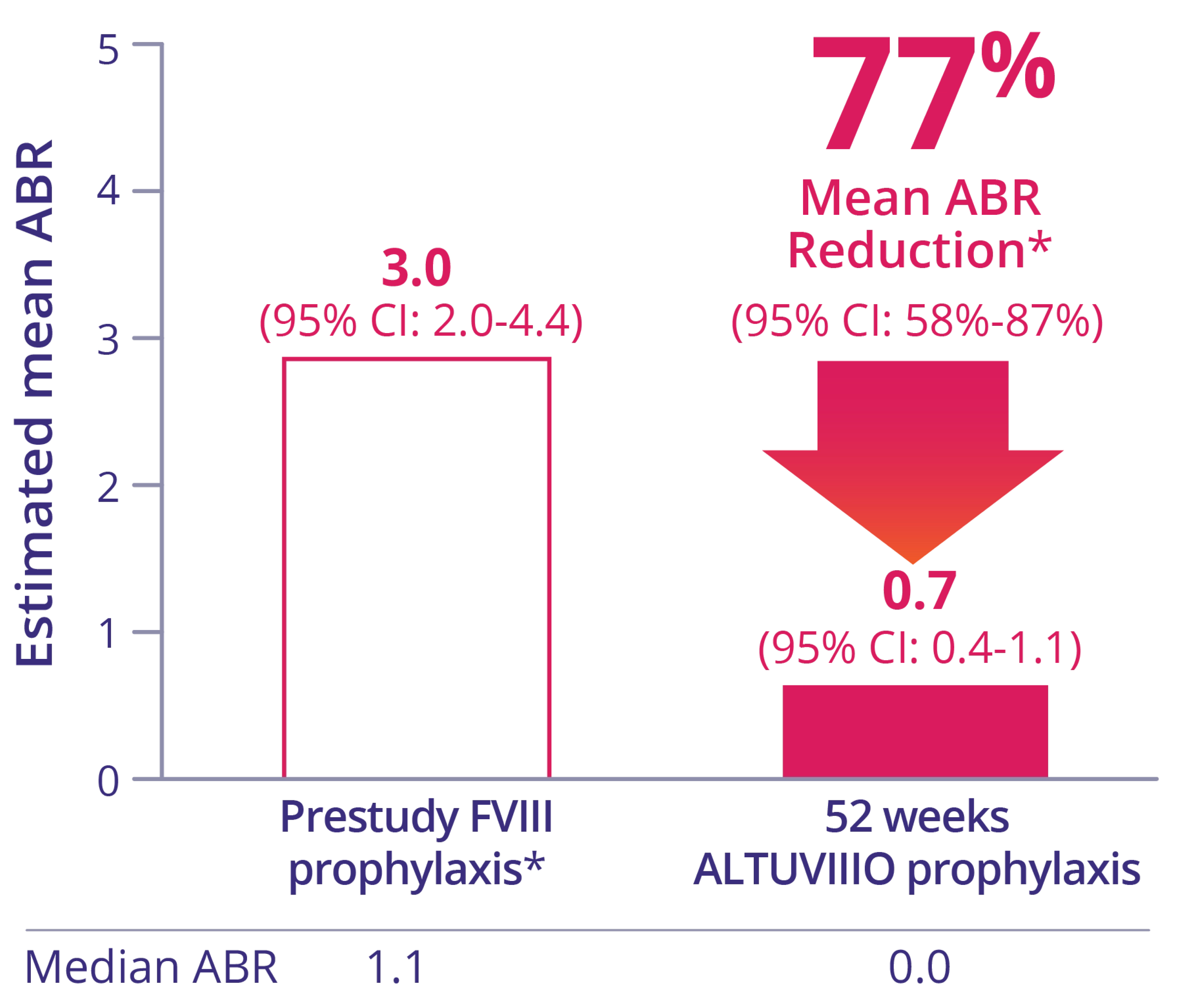
* Data from prospective observational study.1
‡ Based on treated bleeds.1
Intrapatient ABR comparison (n=26) prior Factor VIII On-Demand Group1,2
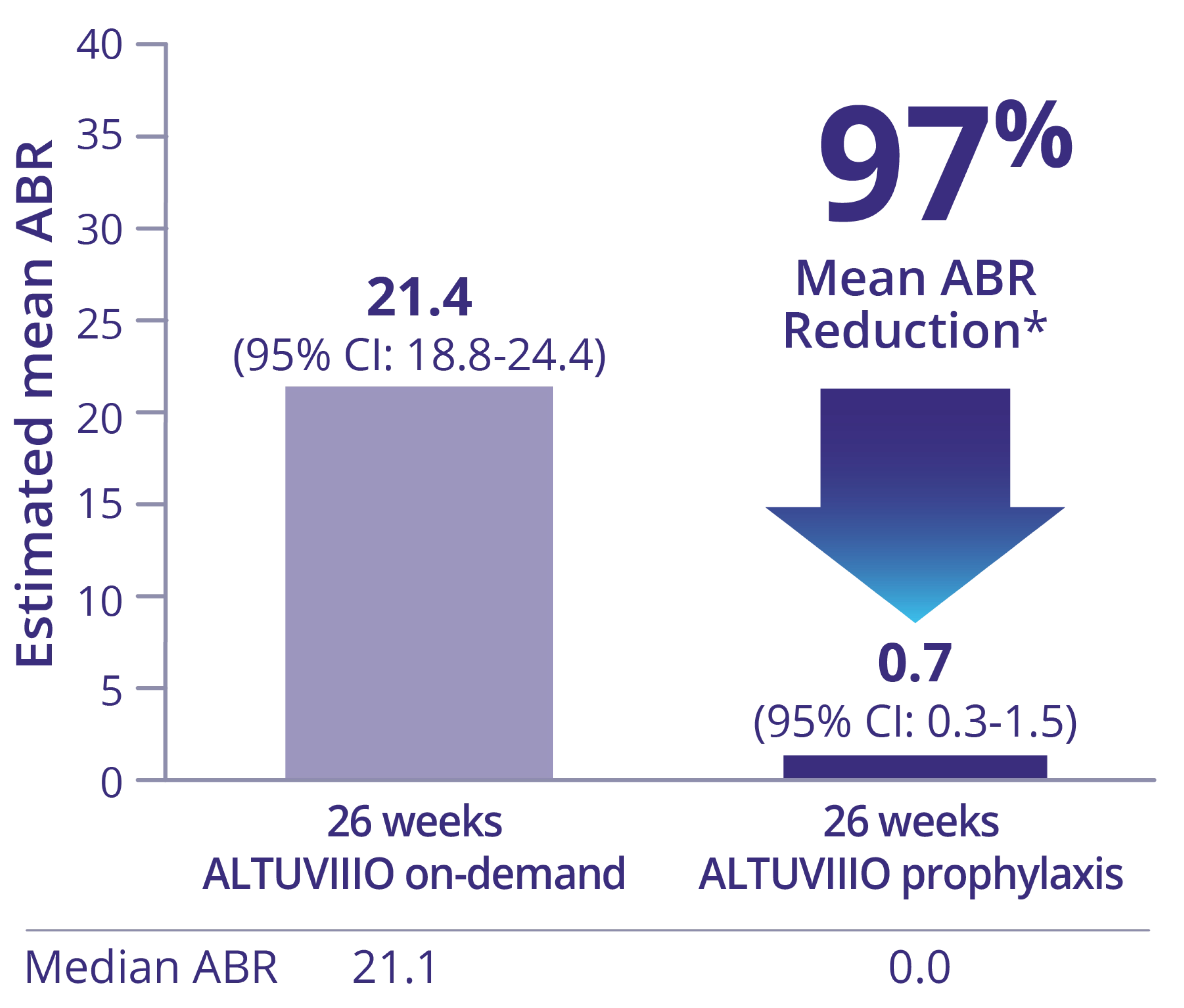
Mean number of bleeding episodes reported during the 12 months prior to the study was 35.7 (SD: 22.2) in the Prior Factor VIII On-Demand Group.1
* Based on treated bleeds.1
Proven joint bleed protection and improved joint health1,2

Prior Factor VIII Prophylaxis Group* (n=128)
0.0 Median AjBR (Q1, Q3: 0.0, 1.0)
Mean AjBR†: 0.5 (95% CI: 0.4, 0.7)
52 weeks of prophylaxis

Prior Factor VIII On-Demand Group* (n=26)
0.0 Median AjBR (Q1, Q3: 0.0, 0.0)
Mean AjBR†: 0.6 (95% CI: 0.3, 1.5)
26 weeks of prophylaxis
* Based on treated bleeds.1
† Based on negative binomial model.2

Improved target joints and joint health1,2
Hemophilia Joint Health Score (HJHS) showed improvement1:
−1.5 (−2.7 to −0.4) points in subjects on prophylaxis at Week 52 vs baseline§
- HJHS is a validated tool used to measure joint health. The open-label nature of the study may impact interpretation of the results
‡ All patients with target joints at baseline (defined as ≥3 spontaneous bleeding episodes in a major joint which occurred in a consecutive 6-month period) achieved resolution of all target joints (45/45, 100%) with 12 months of prophylactic treatment with ALTUVIIIO (defined as ≤2 bleeding episodes in the target joint in 12 months).1,2
§ Mean change in total score from baseline to Week 52.1
Most patients experienced ZERO bleeds when treated with ALTUVIIIO2
Patients with zero bleeds*
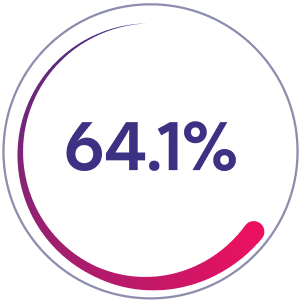
Prior Factor VIII Prophylaxis Group
64.1% of patients (n=82/128) had zero bleeds in 52 weeks
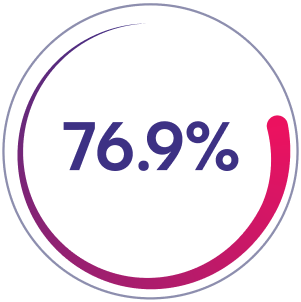
Prior Factor VIII On-Demand Group
76.9% of patients (n=20/26) had zero bleeds in 26-week prophylaxis phase
Patients with zero joint bleeds*
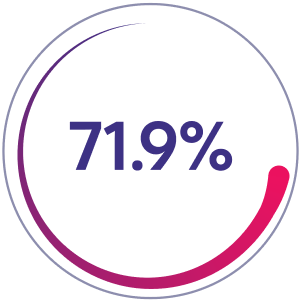%201.png)
Prior Factor VIII Prophylaxis Group
71.9% of patients (n=92/128) had zero joint bleeds in 52 weeks
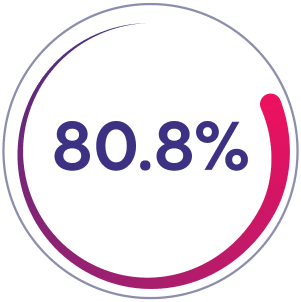
Prior Factor VIII On-Demand Group
80.8% of patients (n=21/26) had zero joint bleeds in 26-week prophylaxis phase
* Based on treated bleeds.2
Effective on-demand bleed management with only 1 infusion2
Treatment of bleeding episodes in the XTEND-1 trial2

96.7% of bleeds* resolved with 1 infusion (n=350/362)
* One 50 IU/kg infusion of ALTUVIIIO (350/362 bleeds). Substantial majority of bleeding events in XTEND-1 (74%, 268/362) occurred during on-demand treatment in the Prior Factor VIII On-Demand Group.1
Pain and physical health scores from patient-reported outcomes
In patients on ALTUVIIIO prophylaxis who switched from prior prophylaxis in the XTEND-1 trial1
PROMIS pain intensity*
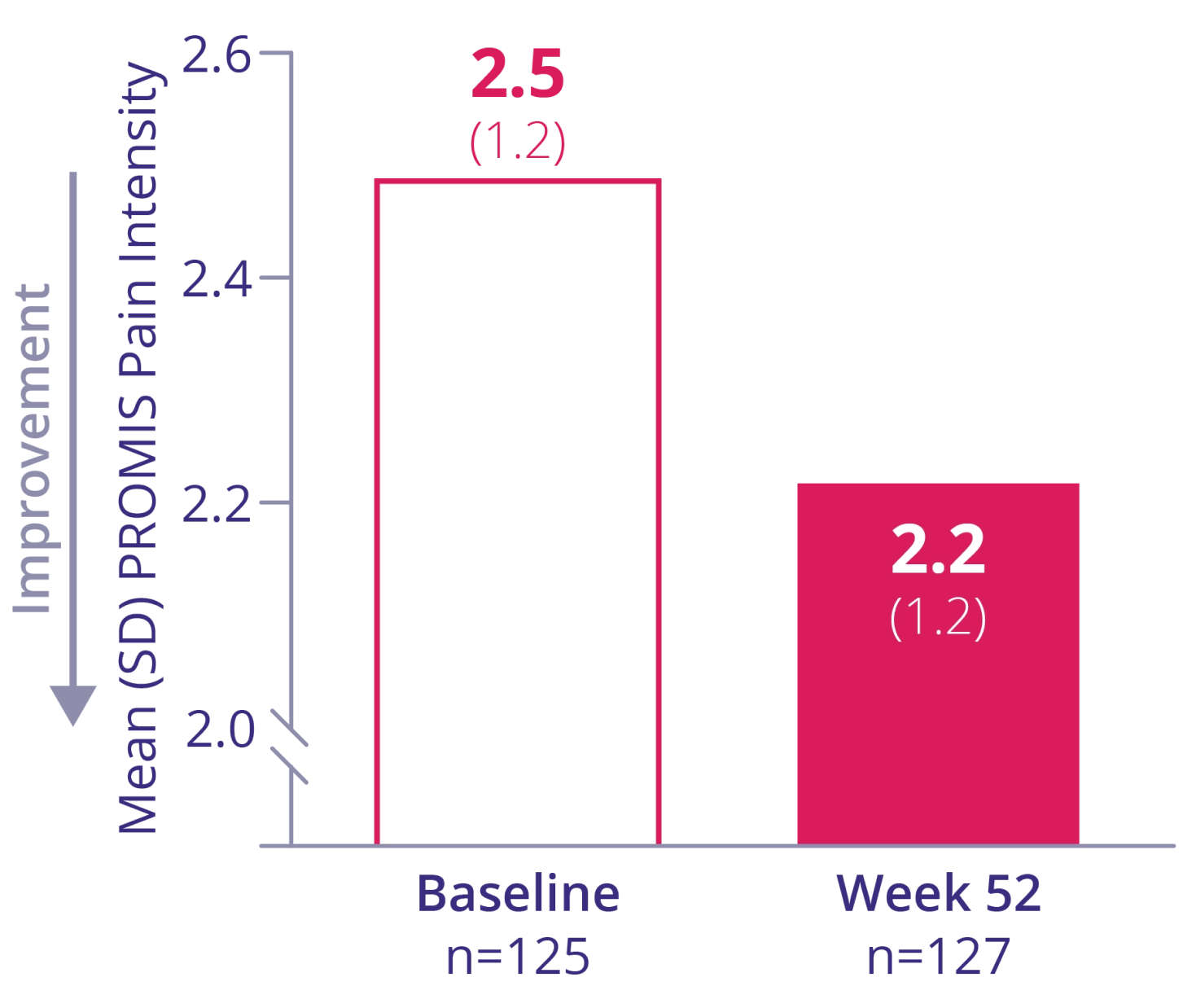
REDUCTION IN PAIN
Mean change†:
-0.2 (95% CI: -0.4 to 0.0)
HAEM-A-QoL Physical-Health Score in patients ≥17 years of age1†
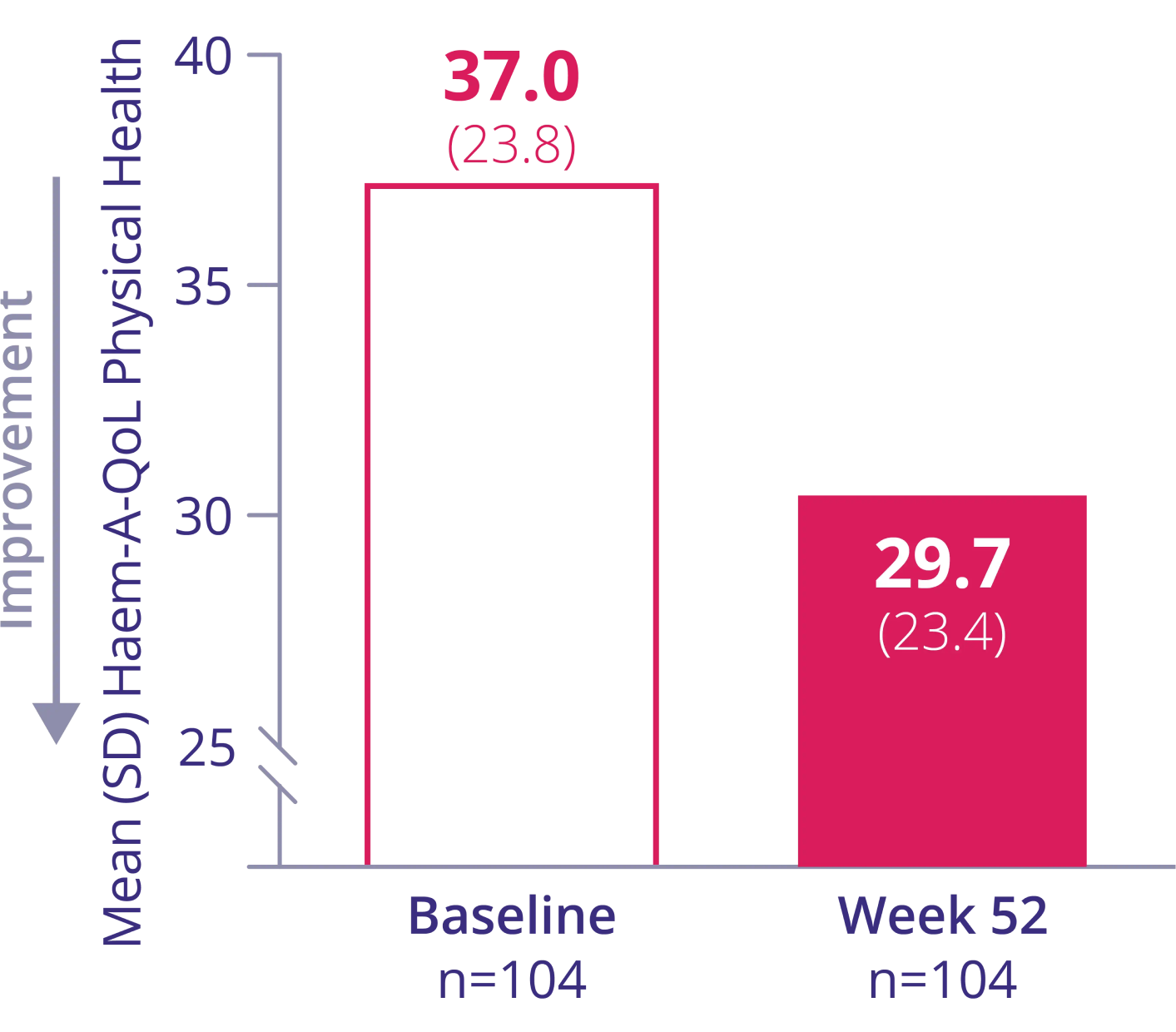
IMPROVED PHYSICAL HEALTH
Mean change†:
-6.7 (95% CI: -10.1 to -3.4)
• A lower score represents an overall improvement in these measures
XTEND-1 was a single-arm study, which may impact the interpretation of patient-reported outcome findings. The absence of a comparator arm may result in an under- or overestimation of treatment effect. Patient-reported nature of data may impact the reliability of findings. The PROMIS instrument has not been validated in hemophilia patients.1
Study design: Patient-reported outcomes of pain intensity and physical functioning were evaluated in patients receiving ALTUVIIIO prophylaxis in the prior Factor VIII prophylaxis group. The PROMIS Pain Intensity 3a instrument was used to assess pain, specifically, the first question that rates the worst pain experienced during the last 7 days. Physical functioning was assessed in patients ≥17 years old using the Physical-Health Score of Haem-A-QoL, which evaluated factors such as painful swellings, presence of joint pain, pain with movement, difficulty walking far, and needing more time to get ready.1
* Worst pain experienced during the last 7 days (1=no pain; 2=mild pain; 3=moderate pain; 4=severe pain; 5=very severe pain).1
† LS mean based on mixed-effect model with repeated measures (MMRM) with visit as fixed effect, and baseline score as a covariate.1
XTEND-1 treatment preference: participant-reported outcomes
Patient preference was evaluated using a 2-item questionnaire on perceived impact of treatment at the end of the study3
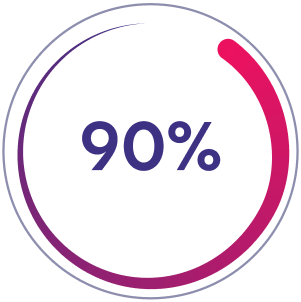
Arm A: Prophylaxis Group
90% preferred ALTUVIIIO vs prior prophylaxis treatment (n=117/130)

Arm B: On-Demand Group
100% preferred ALTUVIIIO vs prior on-demand treatment (n=25/25)
| Among the 142 participants who preferred treatment with ALTUVIIIO, the most common reasons for preference were3: | |
| Less frequent treatment | 81% (n=115) |
| Bleeds reduction | 69% (n=98) |
| Feel better protected | 64% (n=91) |
XTEND-1 was an open-label, single-arm study, which may impact the interpretation of patient-reported outcome findings. Patient-reported nature of data may impact the reliability of findings. The treatment preference questionnaire used is not a validated instrument.
In XTEND-1, most participants indicated they preferred treatment with ALTUVIIIO vs their previous hemophilia treatment option3
Indication
ABR=annualized bleed rate; AjBR=annualized joint bleed rate; CI=confidence interval; FVIII=Factor VIII; Haem-A-QoL=Haemophilia Quality of Life Questionnaire for Adults; IU=international unit; LS=least squares; PROMIS=Patient-Reported Outcomes Measurement Information System; Q1=25th percentile; Q3=75th percentile; SD=standard deviation.
References: 1. von Drygalski A, et al. N Engl J Med. 2023;388(4):310-318. 2. ALTUVIIIO Prescribing Information. Bioverativ Therapeutics Inc. Waltham, MA. 3. Data on file, June 2022.