No Factor VIII inhibitors were detected during the XTEND-Kids study1*
Primary endpoint: Incidence of neutralizing antibodies to Factor VIII2

0.0% (95% Cl: 0.0-4.9)2
Formation of inhibitors to Factor VIII is possible following administration of ALTUVIIIO.1
* Defined as an inhibitor of ≥0.6 BU/mL and confirmed by a second test result from a separate sample drawn 2 to 4 weeks following the date of the original sample.2
Powerful bleed protection with a once-weekly infusion1
In the XTEND-Kids trial:
ALTUVIIIO demonstrated proven bleed protection in children <12 years (n=72)*

0.0 Median ABR
(Q1, Q3: 0.0, 1.0)

0.6 Mean ABR
(95% Cl: 0.4-0.9)
* Based on treated bleeds.
Most patients experienced zero bleeds when treated with ALTUVIIIO prophylaxis1
In the XTEND-Kids trial*:
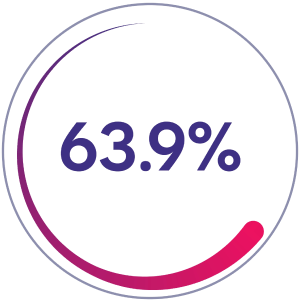
Zero bleeds
63.9% of patients (n=46/72) had zero bleeds
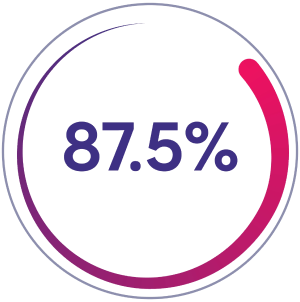
Zero spontaneous bleeds
87.5% of patients (n=63/72) had zero spontaneous bleeds
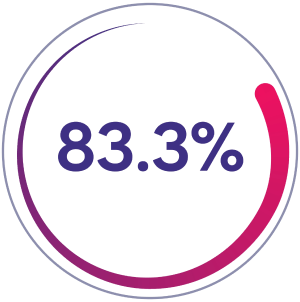
Zero joint bleeds
83.3% of patients (n=60/72) had zero joint bleeds
* Based on treated bleeds.
Effective on-demand bleed management with only 1 infusion1
Treatment of bleeding episodes in the XTEND-Kids trial1,2

95.3% of bleeds* resolved with 1 infusion (n=41/43)
* Percentage based on the number of treated bleeding episodes in the sensitivity analysis set, which excluded two patients.1,2
Indication
ABR=annualized bleed rate; BU=Bethesda unit; CI=confidence interval; IU=international unit; Q1=25th percentile; Q3=75th percentile.
References: 1. ALTUVIIIO Prescribing Information. Bioverativ Therapeutics Inc. Waltham, MA. 2. Malec L, et al. Efanesoctocog Alfa Prophylaxis for Previously Treated Patients <12 Years of Age With Severe Hemophilia A. Oral presentation at: 2023 International Society on Thrombosis and Haemostasis (ISTH); June, 2023; Montreal, Canada.