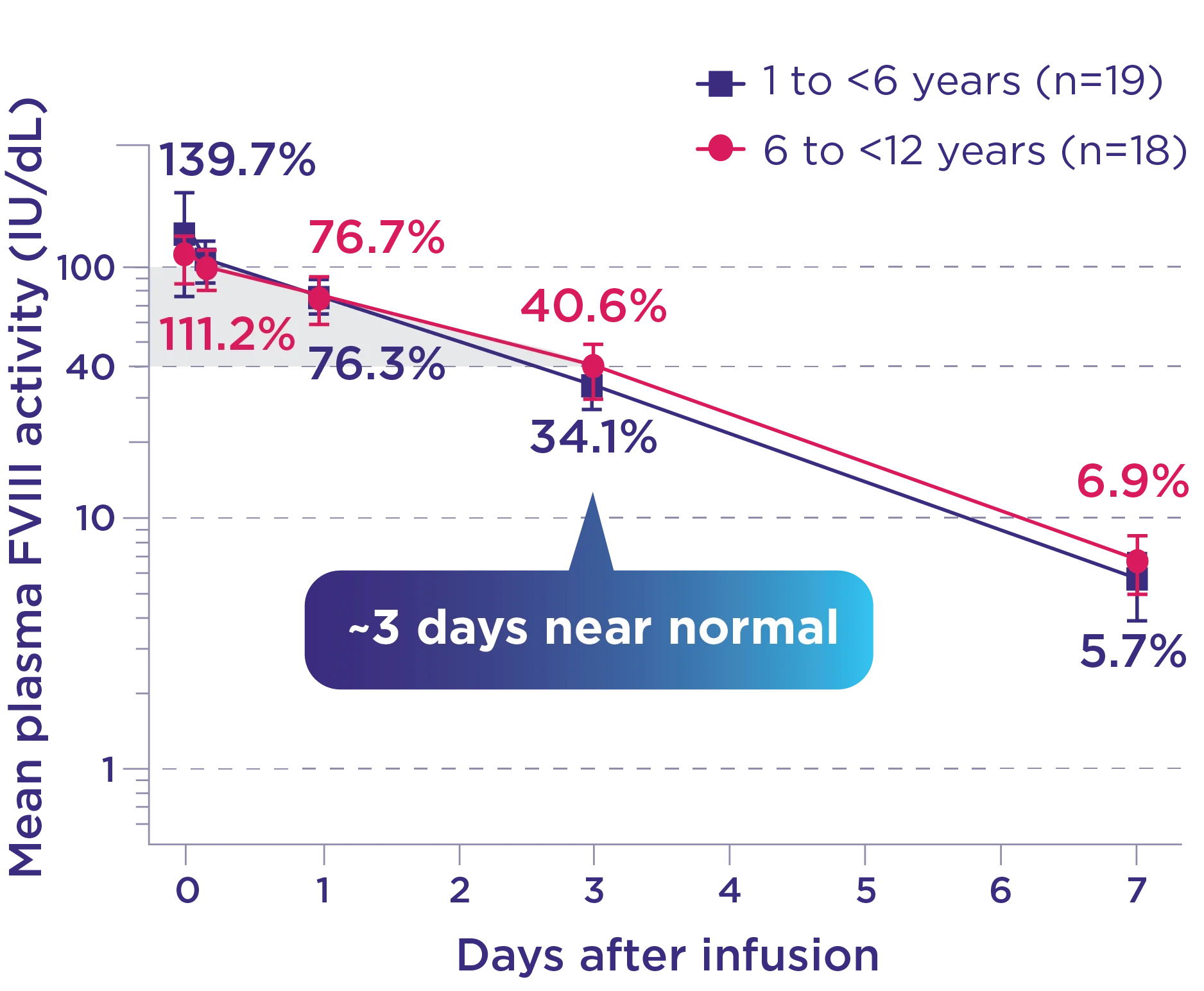
Deliver ~3 days near normal for your pediatric patients1
In children <12 years, ALTUVIIIO provided mean steady-state Factor VIII levels >40% for ~3 days and an unprecedented trough level of 10% at ~Day 71
Phase 3 XTEND-Kids FVIII activity after first dose in PK subgroup2*
End of study population (N=73)1
Once-weekly ALTUVIIIO delivered Factor VIII trough levels†:
- 10.9% in children <6 years
- 16.5% in children 6 to <12 years
* Data from this subgroup analysis includes 37 children (<12 years) receiving a weekly IV infusion of 50 IU/kg ALTUVIIIO. PK parameters shown are based on plasma FVIII activity measured by the aPTT-based one-stage clotting assay.1
† Mean steady-state trough levels were computed using available measurements at Week 52/end of study PK sampling visit for the pediatric population in the XTEND-Kids study (n=72).1
ALTUVIIIO offered a mean half-life of 40.2 hours in children <12 years—the longest of any Factor VIII replacement therapy1-11‡
‡ Comparison of data from XTEND-Kids with half-life data recorded in Prescribing Information of approved Factor VIII replacement therapies.
Indication
References: 1. ALTUVIIIO Prescribing Information. Bioverativ Therapeutics Inc. Waltham, MA. 2. Data on file, May 2023. 3. Advate Prescribing Information. Takeda Pharmaceuticals U.S.A., Inc. Lexington, MA. 4. Adynovate Prescribing Information. Takeda Pharmaceuticals U.S.A., Inc. Lexington, MA. 5. Afstyla Prescribing Information. CSL Behring LLC. Kankakee, IL. 6. Eloctate Prescribing Information. Bioverativ Therapeutics Inc. Waltham, MA. 7. Esperoct Prescribing Information. Novo Nordisk Inc. Plainsboro, NJ. 8. Kovaltry Prescribing Information. Bayer HealthCare LLC. Whippany, NJ. 9. Novoeight Prescribing Information. Novo Nordisk Inc. Plainsboro, NJ. 10. Nuwiq Prescribing Information. Octapharma USA, Inc. Paramus, NJ. 11. Xyntha Prescribing Information. Wyeth Pharmaceuticals LLC. Philadelphia, PA.