For your appropriate patients' basal insulin journey,
Start with Toujeo® Max SoloStar®
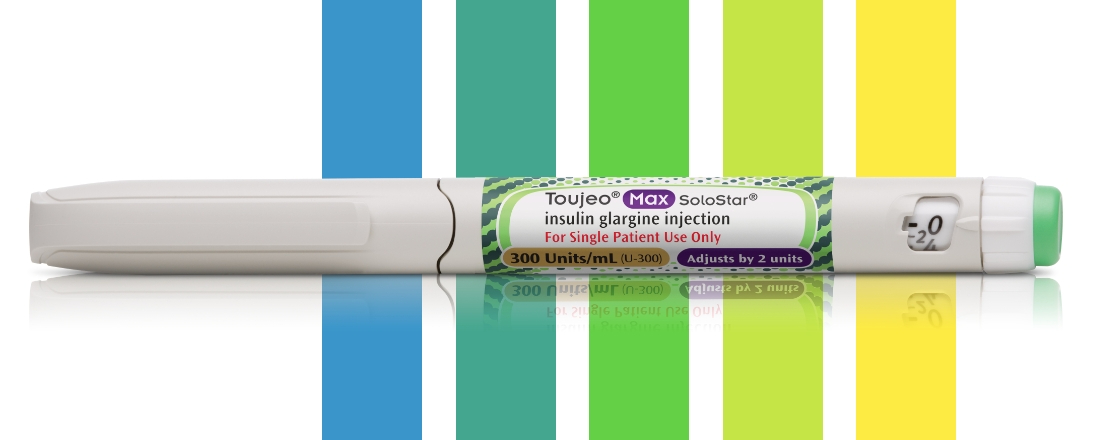
Toujeo® Max SoloStar® is the highest capacity once-daily basal insulin pen on the market with 900 units and a 56-day shelf life. The pen is capable of delivering 20 to 160 units per injection and is recommended for appropriate patients who require at least 20 units per day.
The recommended starting dose of Toujeo® U-300 (insulin glargine) injection 300 Units/mL in insulin-naive patients with type 2 diabetes is 0.2 units per kilogram of body weight once daily.1
Pay no more than $35/month†
See available savings for eligible patients with and without prescription insurance.
Add to your Home Screen
For iPhone users: With the HCP Connection Portal page open in Safari, select the middle icon with an arrow located below the search bar. Scroll down and press “Add to Home Screen.” In the following window, you can rename the webpage as you’d like it to appear on your home screen, then select “Add” in the top right corner. The HCP Connection Portal is now on your home screen so you can access it directly anytime.
For Android users: With the HCP Connection Portal page open in Chrome, select the three dot icon in the top right. Scroll down and press “Add to Home Screen.” In the popup window, you can rename the webpage as you’d like it to appear on your home screen, then press “Add.” The HCP Connection Portal is now on your home screen so you can access it directly anytime.
Important Safety Information
T2DM, type 2 diabetes mellitus.
†Eligibility Restrictions & Offer Terms for Your Patients:
Sanofi Insulins Co-pay Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Sanofi Insulins Co-pay Savings Program at (866) 255-8661 (8:00 am-8:00 pm EST, Monday-Friday).
Insulins Valyou Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is only valid for those who are uninsured or those who are insured by a prescription plan but are not using such insurance and will be paying the full retail price for the medication. Void where prohibited by law. The Savings Program applies to the cost of medication. There are other relevant costs associated with overall treatment. You may not submit claims for reimbursement to any third-party payor, including any government healthcare plan (e.g., Medicare, Medicaid, DOD, VA, TRICARE) or similar federal or state programs for Sanofi Insulin prescriptions when using this Program. You may not seek to have your out-of-pocket costs or the full retail price of the Sanofi Insulin count toward your deductible, true-out-of-pocket (TrOOP), maximum out-of-pocket (MOOP), or any other out-of-pocket caps associated with any insurance coverage. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Insulins Valyou Savings Program at (833) 813-0190 (8:00 am-8:00 pm EST, Monday-Friday).
§OHIO HCPs: Prescription drug samples may only be provided to a prescriber whose practice is licensed as a Terminal Distributor of Dangerous Drugs ("TDDD") or qualifies as exempt under Ohio law. For more information on the State of Ohio Board of Pharmacy Prescriber licensure requirements, how to obtain a TDDD license, and reasons for exemptions, please go to: http://www.pharmacy.ohio.gov/Licensing/TDDD.aspx
Reference:
1. Toujeo Prescribing Information. Sanofi.