FLUBLOK HAS BEEN SHOWN TO HELP PROTECT PATIENTS AGED 18+ AGAINST FLU AND ITS COMPLICATIONS1,2
RECOMBINANT TECHNOLOGY
FLUBLOK COMBINES THE ADVANTAGES OF RECOMBINANT TECHNOLOGY WITH A HIGHER DOSE2,3

3x THE ANTIGEN1,2,4
Flublok contains 3x the hemagglutinin (HA) antigen content of cell- and egg-based standard-dose flu vaccines, which has been linked to greater immunogenicity vs standard-dose flu vaccines.*
ELIMINATES RISK OF ANTIGENIC MISMATCH DURING MANUFACTURING3,5
The only flu vaccine produced with recombinant technology, Flublok ensures identical antigenic match with FDA-selected flu strains. Recombinant technology eliminates the risk of mutations that can occur with cell- or egg-based vaccines.
MAY PROVIDE CROSS-PROTECTION3
Recombinant technology leads to a broader immune response and may provide cross-protection against drifted strains during mismatched seasons.†
MAY INDUCE A MORE ROBUST IMMUNE RESPONSE6
Vaccination with a higher-dose recombinant flu vaccine may lead to a greater antibody response vs cell- and egg-based standard-dose vaccines.

*Flublok contains 45 micrograms (mcg) of HA per strain vs 15 mcg of HA per strain in a standard-dose influenza vaccine.1,2,4
†Flublok is produced using a novel production platform in which recombinant HA is expressed in insect cells using a baculovirus expression vector system (BEVS). Recombinant HA antigens produced using BEVS have been shown to induce significantly higher levels of broadly cross-reactive antibodies against highly conserved regions of HA compared with egg-derived vaccines, which may potentially protect against drift-variant influenza viruses.3
‡According to the CDC, getting a flu vaccine will not give you the flu.8
CDC=Centers for Disease Control and Prevention; FDA=US Food and Drug Administration.
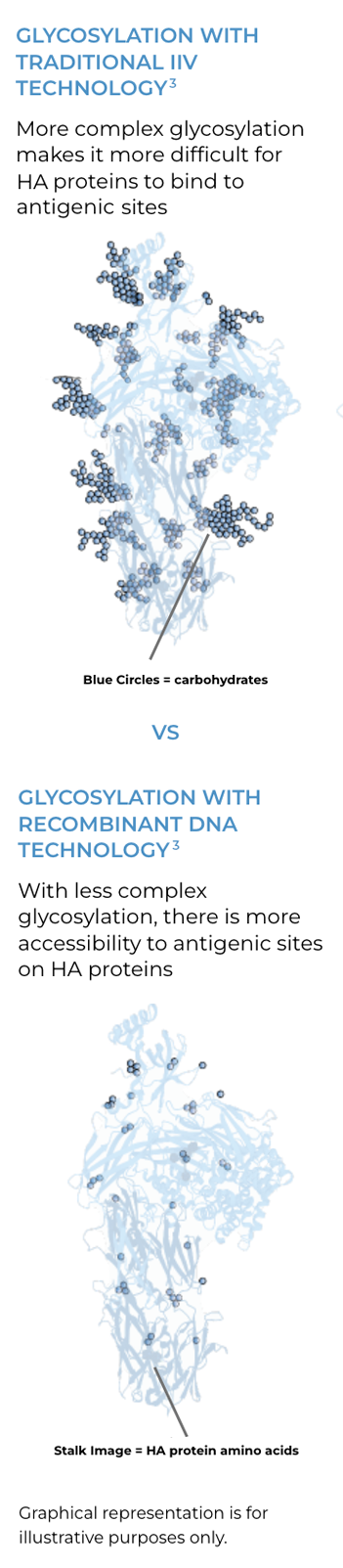
CROSS-PROTECTION MAY BE A RESULT OF SIMPLER GLYCOSYLATION BECAUSE RECOMBINANT DNA TECHNOLOGY ALLOWS FOR BROADER ACCESS TO CONSERVED ANTIGENIC SITES3
Glycosylation occurs during viral replication and in the vaccine manufacturing process, blocking access to antigenic sites on HA proteins, which are necessary for developing an immune response. Complex glycosylation interferes with antigenic site access.3,9

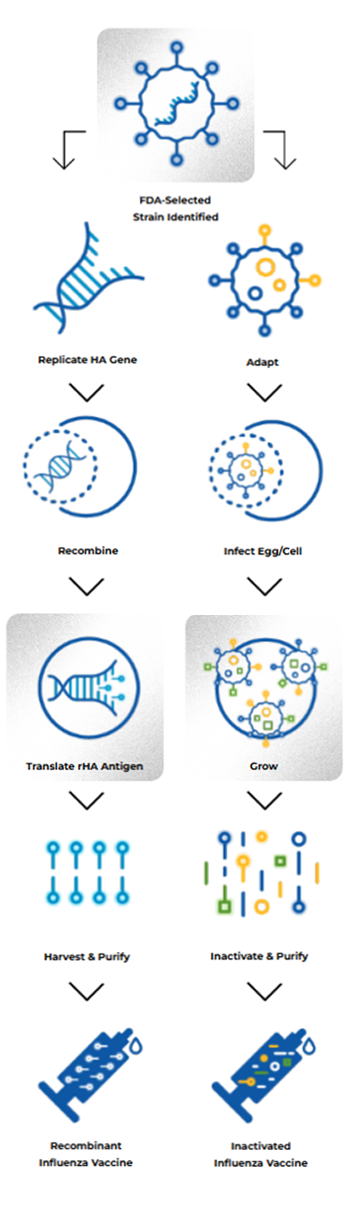
HOW IT’S MADE
RECOMBINANT DNA TECHNOLOGY ELIMINATES THE NEED TO GROW INFLUENZA VIRUS AND AVOIDS THE POSSIBILITY OF MUTATION OR ADAPTATION2

PIVOTAL TRIAL IN ADULTS AGED 50+:
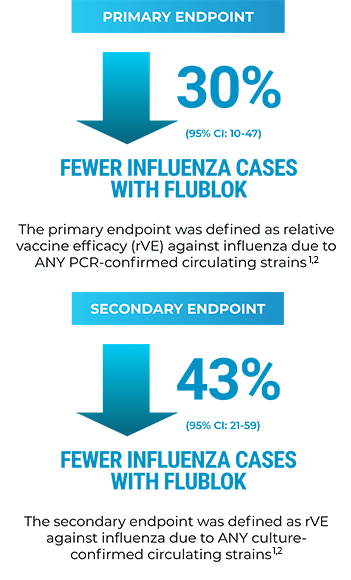
IN A RANDOMIZED, CONTROLLED TRIAL VS FLUARIX: 30% RELATIVE REDUCTION IN PCR-CONFIRMED FLU IN ADULTS AGED 50+1,2
Study Design
- Phase 3-4 randomized, controlled trial in adults aged 50+ (N≈9,000) during the 2014-2015 influenza season in which A (H3N2) was predominant and antigenically mismatched1,2
- Patients were randomized 1:1 to receive Flublok (quadrivalent) or Fluarix (quadrivalent)1,2
- Patients with comorbidities who received Flublok: insulin-dependent diabetes: 3.9% (170); non-insulin-dependent diabetes: 10.8% (469); atherosclerotic cardiovascular disease: 30.5% (1320); condition requiring statin lipid-lowering therapy: 27.6% (1194); condition requiring thiazide diuretic: 7.7% (332); chronic obstructive pulmonary disease: 3.3% (144); acid reflux or peptic ulcer disease: 14.5% (629); depression: 18.2% (788)2
The efficacy of Flublok (quadrivalent) is relevant to Flublok (trivalent) because both vaccines are manufactured using the same process and have overlapping compositions.1

Flublok prevented more cases of influenza than a standard-dose vaccine and satisfied the primary criterion for noninferiority and the prespecified exploratory superiority criterion.2
SAFETY IN ADULTS AGED 50+
- Comparable safety profile to a standard-dose quadrivalent inactivated influenza vaccine in adults aged 50+2
- Most common adverse events (≥10%) in the Flublok group in adults aged 50-642:
- Injection-site reactions: tenderness (37%), pain (32%)
- Systemic adverse reactions: headache (17%), fatigue (13%), muscle pain (11%)

PIVOTAL TRIAL IN PATIENTS AGED 18-49:
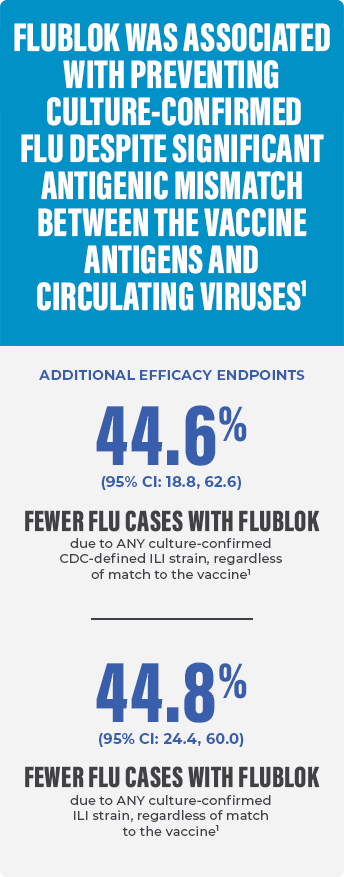
EFFECTIVE FLU PROTECTION, EVEN IN A SEASON WITH SIGNIFICANT ANTIGENIC MISMATCH, IN PATIENTS AGED 18-49 YEARS1,10
Study Design
- Randomized, observer-blind, placebo-controlled trial to evaluate the protective efficacy and safety of Flublok (trivalent) against influenza in 4648 adults aged 18-49 years1,10
- The study was undertaken during the 2007-2008 influenza season when there was significant mismatch between vaccine antigens and circulating viruses10
- Primary endpoint: CDC-defined influenza-like illness (ILI) defined by presence of documented fever ≥100 °F plus either sore throat or cough with positive culture for an influenza virus strain antigenically resembling a strain represented in Flublok. Vaccine efficacy against antigenically matched culture-confirmed CDC-ILI could not be determined reliably because 96% of the influenza isolates obtained were not antigenically matched to the strains represented in the vaccine1

IMMUNOGENICITY IN PATIENTS AGED 9-17
FLUBLOK DEMONSTRATED NON-INFERIOR IMMUNE RESPONSE IN PATIENTS AGED 9-17 COMPARED TO ADULTS AGED 18-491,11
Study Design
- A phase 3 non-randomized, open-label, uncontrolled, multicenter study of 1308 participants aged 9 to 49 years1
- Primary objective was to demonstrate that vaccination with Flublok induced an immune response in children and adolescents aged 9 to 17 that was non-inferior to responses induced by Flublok in adults aged 18 to 49 for 4 viral strains at Day 29 post-vaccination1
- Immune response was assessed by hemagglutination inhibition (HI), geometric mean titers (GMTs), and seroconversion (SCR) rates1

- The non-inferiority of HI immune responses induced by Flublok (quadrivalent) in patients aged 9 to 17 relative to patients aged 18 to 49 was demonstrated for all 4 strains (A/H1N1, A/H3N2, B/Victoria, and B/Yamagata)1*
- Non-inferiority was based on prespecified criteria (lower limit of the 2-sided 95% CIs of the ratios of GMTs between age groups [9-17 years/18-49 years] >0.667; lower limit of the 2-sided 95% CI of the difference in SCR rates > −10 at Day 29 post-vaccination)1
*Effectiveness of Flublok (quadrivalent) in children aged 9 to 17 is based on comparison to adults aged 18 to 49. The data for Flublok (quadrivalent) is relevant to Flublok (trivalent) because both vaccines are manufactured using the same process and have overlapping compositions.1

REAL-WORLD EVIDENCE
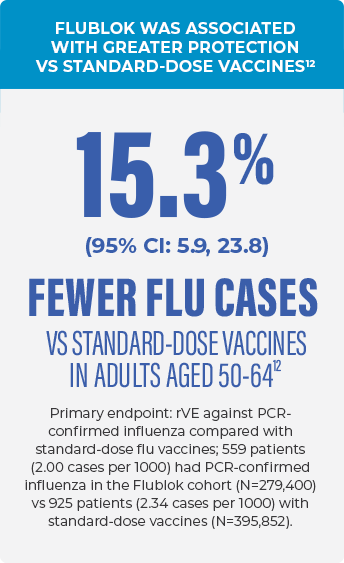
FLUBLOK WAS STUDIED AGAINST STANDARD-DOSE VACCINES IN THE LARGEST RANDOMIZED REAL-WORLD FLU EFFECTIVENESS STUDY TO DATE12,13
Study Design
- Modified cluster randomized observational study to evaluate Flublok (quadrivalent) vs quadrivalent standard-dose influenza vaccines12
- Study population: 1,630,328 members of the Kaiser Permanente Northern California (KPNC) healthcare system aged 18-64 years12
- Evaluated over 2 flu seasons: 2018-2019 influenza season, when A (H1N1) was predominant until March 2019, when A (H3N2) viruses became predominant; and the 2019-2020 influenza season, when A (H1N1) was predominant with B cocirculation14-16
- Patients aged 50-64 with comorbid conditions: asthma: 14% (96,307); diabetes: 18% (119,430); chronic obstructive pulmonary disease: 2% (13,357); coronary heart disease: 4% (25,496)12
The efficacy of Flublok (quadrivalent) is relevant to Flublok (trivalent) because both vaccines are manufactured using the same process and have overlapping compositions.1
Study Limitations12
- Data were limited to two influenza seasons; relative vaccine effectiveness may vary across seasons depending on the vaccine match with circulating strains
- Primary outcome did not include infections in persons who did not undergo PCR testing, which limits generalizability
- Although KPNC has a diverse population, it may not be representative of other populations in the US
- Compliance with the weekly assigned vaccine schedule varied from time to time due to logistical constraints
- The study had limited power to detect a clinically meaningful benefit of Flublok vs standard-dose vaccines with less frequent outcomes, such as hospitalized, PCR-confirmed influenza
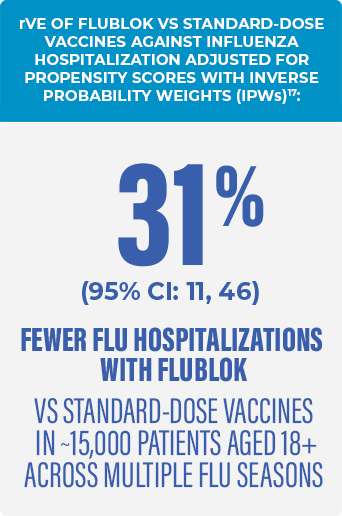
FLUBLOK WAS ASSOCIATED WITH FEWER FLU HOSPITALIZATIONS VS CELL- AND EGG-BASED STANDARD-DOSE VACCINE COMPARATORS17
Study Design
- Retrospective test-negative case-control study in approximately 15,000 patients aged 18+ during the 2018-2019 and 2019-2020 flu seasons to investigate the rVE of Flublok vs standard-dose vaccines against influenza hospitalization17*
- In the primary analysis, Flublok was evaluated against standard-dose flu vaccines, including17,18:
- Cell-based: Flucelvax
- Egg-based: Fluarix, Afluria, FluLaval, and Fluzone® (Influenza Vaccine)
*Flublok (quadrivalent vaccine) was evaluated against quadrivalent standard-dose vaccines. The efficacy of Flublok (quadrivalent formulation) is relevant to Flublok (trivalent formation) because both vaccines are manufactured using the same process and have overlapping compositions.1,17
Secondary Endpoints
When adjusted for propensity scores with IPWs17:
- Flublok was associated with providing greater protection against influenza hospitalization vs standard-dose vaccines for the following populations17:
- Female sex: 37% rVE (95% CI: 13, 54)
- Age 18-64 years: 28% rVE (95% CI: 3, 46)
- No high-risk condition: 60% rVE (95% CI: 29, 78)
- Flublok was associated with providing greater (non–statistically significant) protection against influenza hospitalization vs standard-dose vaccines for the following populations17:
- Male sex: 23% rVE (95% CI: -14, 48)
- Age ≥65 years: 17% rVE (95% CI: -36, 48)
- High-risk condition: 20% rVE (95% CI: -7, 40)
Study Strengths and Limitations17
- The demographics of the study population were representative of the adult population of Allegheny County, with 79% being white and 51% being female, which contributes to generalizability
- The University of Pittsburgh Medical Center hospitals in central and southwestern Pennsylvania are part of an integrated health system, with regular uploads of vaccination data from the state immunization registry; vaccination status was verified through the state registry with a specific data request
- Although the electronic medical records (EMR) system of University of Pittsburgh Medical Center hospitals in central and southwestern Pennsylvania is robust, if vaccinations were not captured in the EMRs or state registry, they were classified as unvaccinated
- Because data focused on hospitalizations, there may have been milder cases of influenza that were not captured in the EMRs because they didn’t require medical care
- There is a possibility of selection bias among those who received influenza testing; for instance, clinicians might preferentially test unvaccinated subjects, which would increase the proportion of unvaccinated cases
- While a relatively large cohort of adults is included in this study, the sample size of standard-dose flu vaccine recipients may have been inadequate to detect meaningful rVE estimates for specific subgroups
2019-2020 RETROSPECTIVE OBSERVATIONAL COHORT STUDY:
FLUBLOK WAS ASSOCIATED WITH SIGNIFICANTLY FEWER INFLUENZA HOSPITAL ENCOUNTERS COMPARED WITH STANDARD-DOSE INFLUENZA VACCINES19
Study Design
- Retrospective observational cohort study using real-world data from Medicare claims to analyze the rVE of US-licensed influenza vaccines in preventing influenza hospital encounters among beneficiaries aged 65+; 12.7 million adults aged 65+ were eligible for analysis19
- The efficacy of Flublok (quadrivalent) is relevant to Flublok (trivalent) because both vaccines are manufactured using the same process and have overlapping compositions1
Relative Vaccine Effectiveness (rVE) Against Influenza Hospital Encounters (95% CI)19
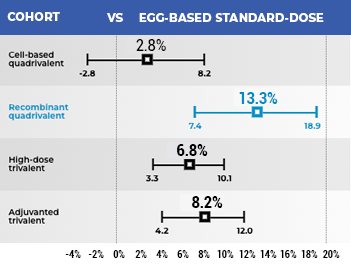
The data above represents 1 of 3 primary analyses. Two additional primary analyses were conducted19:
- Cell-cultured standard-dose quadrivalent flu vaccine vs egg-based standard-dose quadrivalent flu vaccine
- Flublok vs egg-based standard-dose quadrivalent flu vaccine
2019-2020 Season Characteristics19
- Influenza A (H1N1) and influenza B (Victoria) were predominating strains with no significant circulation of influenza A (H3N2)
- An H1N1 strain with an amino acid change emerged late in the season and likely did not substantially affect the vaccine efficacy
- Trivalent vaccines contained the influenza B (Victoria) lineage
Study Limitations19
- Lack of access to virological case confirmation may have led to underestimation of the magnitude of differences
- Residual confounding by unmeasured covariates could have affected results
- The observation period was cut off at the end of February to avoid potential bias from the overlap between influenza season and the escalation of the COVID-19 pandemic in the US

FLUBLOK SAFETY
REAL-WORLD SAFETY EVIDENCE
SAFETY PROFILE SUPPORTED IN ONE OF THE LARGEST SAFETY STUDIES OF A FLU VACCINE IN PREGNANT WOMEN, WITH ~15,000 PATIENTS1,20,21
Study Design
- This was an observational, retrospective safety surveillance study of Flublok Quadrivalent in 14,981 pregnant individuals across Northern Hemisphere influenza seasons 2018-2019 and 2019-20201*†‡
- This study represents one of the largest populations of pregnant people to be included in the prescribing information for any FDA-approved flu vaccine1,20,21

*5842 patients, including those with chronic conditions, were exposed to Flublok Quadrivalent during the 28 days prior to conception or during the first trimester. Miscarriage was reported in 464 (3.1%) patients. Among individuals exposed to Flublok Quadrivalent at any time during pregnancy, 1113 pregnancies (7.7%) had infants with major birth defects (56, 360, 381, and 316 among individuals exposed during the 28 days prior to conception, the first trimester, the second trimester, and the third trimester, respectively).1
†Prespecified outcomes included spontaneous abortion and congenital/fetal anomalies. Data were not collected on ectopic pregnancy or elective terminations.1
‡The data for Flublok (quadrivalent) are relevant to Flublok (trivalent) because both vaccines were manufactured using the same process and have overlapping compositions.1


SIMILAR SAFETY PROFILE COMPARED TO STANDARD-DOSE FLU VACCINES IN ADULTS 50-641
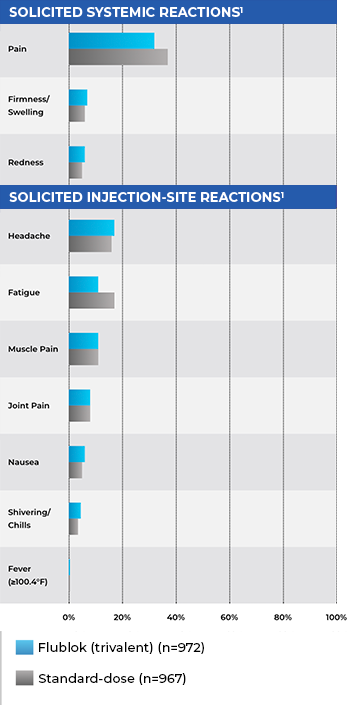
Safety In Adults Aged 50-64: Reactions, Any Grade
- Rates of local and systemic adverse reactions were similar within 7 days of Flublok (trivalent) or standard-dose influenza vaccine administration1
- Pooled data from 2 studies; comparators were Fluzone (trivalent standard-dose formulation) and Afluria (trivalent standard-dose formulation)1
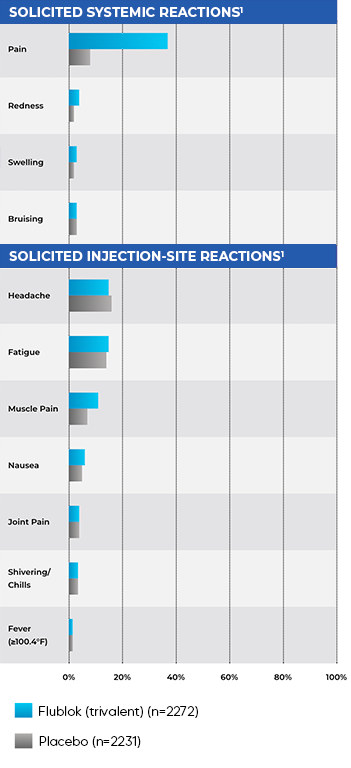
SAFETY PROFILE IN ADULTS AGED 18-49 COMPARED TO PLACEBO10
Safety Trial in Adults Aged 18-49:
- Safety data from a study of 4648 adults randomized to receive Flublok (trivalent; n=2344) or placebo (n=2304)10
- Systemic symptoms following vaccination were similar between people receiving Flublok and placebo10
- The most frequently reported systemic symptoms following vaccination were headache (15% with Flublok vs 16% with placebo) and fatigue (15% with Flublok vs 14% with placebo)10
- 76% of headache complaints were mild
- Flublok was associated with local injection-site pain (37% with Flublok vs 8% with placebo) that was significantly more frequent than after saline placebo1,10
- 94% of all pain complaints after Flublok were rated as mild10
SAFETY IN PATIENTS AGED 9-171
- There were no significant observed differences in safety profile between the 9 to 17-year-old and 18 to 49-year-old populations
- Most common adverse reactions in 9 to 17-year-olds (N=608-618):
- Injection-site reaction: pain (34.4%)
- Systemic reactions: myalgia (19.3%), headache (18.5%), malaise (16.1%)
Important Safety Information
References: 1. Flublok. Prescribing Information. Protein Sciences Corporation. 2. Dunkle LM, Izikson R, Patriarca P, et al; PSC12 Study Team. N Engl J Med. 2017;376(25):2427-2436. doi:10.1056/NEJMoa1608862 3. Arunachalam AB, Post P, Rudin D. NPJ Vaccines. 2021;6:144. doi:10.1038/s41541-021-00403-7 4. Grohskopf LA, Ferdinands JM, Blanton LH, et al. MMWR Recomm Rep. 2024;73(5):1-25. doi:10.15585/mmwr.rr7305a1 5. Centers for Disease Control and Prevention. Influenza vaccines—United States, 2023–24 influenza season. August 24, 2023. Accessed June 5, 2024. https://archive.cdc.gov/#/details?url=https://www.cdc.gov/flu/professionals/acip/2022-2023/acip-table.htm 6. Liu F, Gross FL, Joshi S, et al. Nat Commun. 2024;15(1):254. doi:10.1038/s41467-023-44551-x 7. Centers for Disease Control and Prevention. Recombinant influenza (flu) vaccine. September 9, 2024. Accessed April 24, 2025. https://www.cdc.gov/flu/vaccine-types/flublok-vaccine.html 8. Centers for Disease Control and Prevention. Key facts about seasonal flu vaccine. September 17, 2024. Accessed April 24, 2025. https://www.cdc.gov/flu/vaccines/keyfacts.html#print 9. Watanabe Y, Bowden TA, Wilson IA, Crispin M. Biochim Biophys Acta Gen Subj. 2019;1863(10):1480-1497. 10. Treanor JJ, El Sahly H, King J, et al. Vaccine. 2011;29(44):7733-7739. doi:10.1016/j.vaccine.2011.07.128 11. Folegatti PM, et al. Lancet Infect Dis. Published online May 21, 2025. doi:10.1016/S1473-3099(25)00153-7 12. Hsiao A, Yee A, Fireman B, Hansen J, Lewis N, Klein NP. N Engl J Med. 2023;389:2245-2255. doi:10.1056/NEJMoa2302099 13. Sanofi. Data on file. 14. Centers for Disease Control and Prevention. Estimated flu-related illness, medical visits, hospitalizations, and deaths in the United States—2019-2020 flu season. October 7, 2022. Accessed June 5, 2024. https://archive.cdc.gov/www_cdc_gov/flu/about/burden/2019-2020/archive-09292021.html 15. California Department of Public Health. Influenza surveillance report 2018-2019 season. December 2019. Accessed June 5, 2024. https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/Immunization/Annual2018-19.pdf 16. California Department of Public Health. Influenza surveillance report 2019-2020 season. June 2021. Accessed June 5, 2024. https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/Immunization/Annual2019-20_FluReport.pdf 17. Zimmerman RK, Nowalk MP, Dauer K, et al. Vaccine. 2023;41:5134-5140. doi:10.1016/j.vaccine.2023.06.056 18. Rhodes RT. BioSupply Trends Quarterly. July 2013. Accessed June 5, 2024. https://www.bstquarterly.com/assets/downloads/BSTQ/Articles/BSTQ_2013-07_AR_Choosing-Influenza-Vaccines.pdf 19. Izurieta HS, Lu M, Kelman J, et al. Clin Infect Dis. 2020;73(11):e4251-e4259. doi:10.1093/cid/ciaa1727 20. Fluarix. Prescribing Information. GlaxoSmithKline. 21. Flucelvax. Prescribing Information. Seqirus Inc. 22. Centers for Disease Control and Prevention. Flu vaccine safety and pregnancy. September 2024. Accessed October 14, 2024. https://www.cdc.gov/flu/vaccine-safety/vaccine-pregnant.html 23. Thompson MG, Kwong JC, Regan AK, et al; PREVENT Workgroup. Clin Infect Dis. 2019;68(9):1444-1453. doi:10.1093/cid/ciy737
