Not only will vaccinating your patients against influenza help to reduce flu illness, but it may also protect against a variety of potentially severe complications.1 Make a recommendation and offer a flu shot to your eligible patients for the upcoming flu season.
Talking to your appropriate patients about the flu vaccine is essential in helping to reduce flu illness and, in some cases, even flu-related hospitalizations.1
WHY RECOMMENDING AND OFFERING THE FLU VACCINE MATTERS
According to a study of data from the 2016 National Internet Flu Survey in 4305 adults aged ≥18 years2:
MORE ADULTS RECEIVED FLU VACCINATION WHEN BOTH RECOMMENDED AND OFFERED BY THEIR HCP
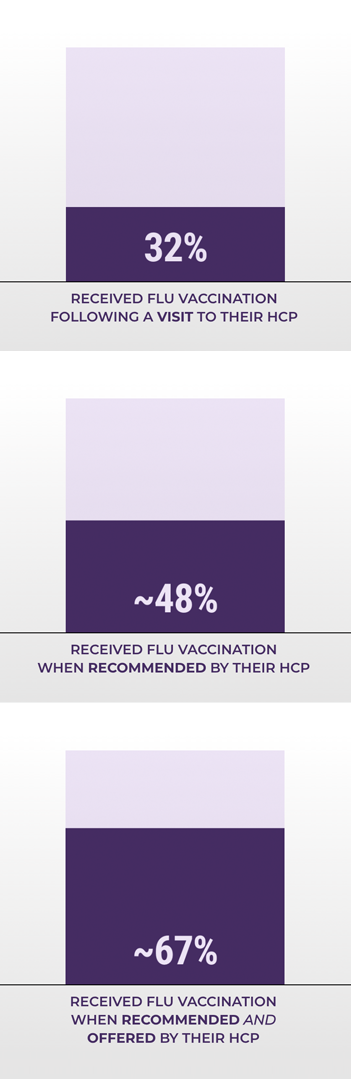
28% did not visit a doctor during the vaccination.
SEASONAL FLU VACCINATION CAN DRAMATICALLY REDUCE INFLUENZA COMPLICATIONS3
| According to CDC estimates for the 2024-2025 flu season, as of the week ending May 17, 2025, there have been3: |
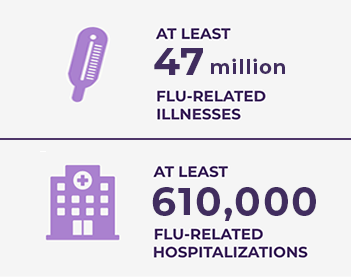
Source: https://www.cdc.gov/flu-burden/php/data-vis/2024-2025.html
CDC=Centers for Disease Control and Prevention.
HOWEVER, THERE IS ROOM FOR IMPROVEMENT IN VACCINE COVERAGE4
| Flu vaccine coverage by age group in the 2024-2025 flu season4 | ||||||
| DEMOGRAPHIC GROUP | 18-29 Years | 30-39 Years | 40-49 Years | 50-64 Years | 65-74 Years | 75+ Years |
| FLU VACCINE COVERAGE 2024-2025 Flu Season | 34.1% Coverage | 36.3% Coverage | 38.9% Coverage | 48.4% Coverage | 69.2% Coverage | 75.6% Coverage |
WHICH PATIENTS HAVE THE GREATEST RISK OF INFLUENZA COMPLICATIONS?
Vaccination for influenza is especially important for those patients at higher risk of developing serious influenza-related complications5:
PEOPLE OF ADVANCED AGE
Although flu infection may carry risk for any age group, the risk of serious complications due to the flu is even higher in people aged 65 and older.6
Of patients with influenza-related hospitalizations in 2023-20247:
- 22% were aged 50-64
- 52% were aged 65+
(According to preliminary CDC influenza-associated hospitalization data as of July 12, 2025.)7
The importance of protection from the flu is well established for people over 65 years, but flu vaccination is also important for those aged 50 to 64. A recent systematic review of relevant studies found that the disease burden of influenza begins to increase well before age 65.8
As well as posing the threat of flu-related health issues, flu infection in the 50-64 age group adds to the economic burden of illness through missed work days, lost productivity, and increased healthcare costs.8
PEOPLE WITH CHRONIC ILLNESSES
Patients who experience influenza infection are at increased risk of developing flu-related complications if they also have pre-existing medical conditions. Examples of common pre-existing conditions include but are not limited to the following categories9:
- Respiratory/pulmonary disease
- Cardiovascular disease
- Type 2 diabetes mellitus
In addition to the CDC recommendations for yearly vaccinations, the American Heart Association, the American Lung Association, the American Diabetes Association, and the National Foundation for Infectious Diseases all recommend that annual flu vaccination is the best protection from the complications of the flu.9
PREGNANT WOMEN
The CDC recommends that women receive a flu shot while pregnant and while breastfeeding to help protect themselves and their babies from the flu.10
Recombinant or inactivated flu vaccines are recommended by the CDC, Advisory Committee on Immunization Practices (ACIP), and American College of Obstetricians and Gynecologists (ACOG) for women who are pregnant or will become pregnant during flu season.1
There is no data to determine the effects of Flublok® (Influenza Vaccine) on breastfed infants or on milk production/excretion.
Healthcare providers are encouraged to enroll women who receive Flublok during pregnancy in Sanofi’s vaccination pregnancy registry by calling 1-800-822-2463.
ADDITIONAL CONSIDERATIONS
When should I administer an influenza vaccine?
Aim to provide influenza vaccination annually by the end of October. But as long as influenza viruses are circulating, flu vaccination should continue to be offered.11
Can the flu vaccine be coadministered?
According to the CDC, vaccines against the flu, COVID-19, and respiratory syncytial virus (RSV) may all be administered at the same visit if the patient is eligible and the timing for each vaccine is right, which may be more convenient for the patient. No clinical trial data are available yet for all 3 vaccines administered at the same time. For additional information, visit the CDC website.12
What if my patient has an egg allergy?
Per the CDC, people with egg allergy may receive an egg-based or non-egg-based vaccine that is otherwise appropriate for their age and health status. All vaccines should be administered in settings in which staff and equipment are available to quickly recognize and treat allergic reactions as needed.13 Fluzone® (Influenza Vaccine), Flublok, and Fluzone® High-Dose (Influenza Vaccine) should not be given to anyone who has had a severe allergic reaction (anaphylaxis) to any component of the vaccine (including egg protein for Fluzone and Fluzone High-Dose). In addition, Fluzone and Fluzone High-Dose should not be given to anyone who has had a severe allergic reaction after a previous dose of any influenza vaccine.14-16
Important Safety Information
References: 1. Grohskopf LA, Ferdinands JM, Blanton LH, Broder KR, Loehr J. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices — United States, 2024-25 influenza season. MMWR Recomm Rep. 2024;73(No. RR-5):1-25. doi:http://dx.doi.org/10.15585/mmwr.rr7305a1 2. Lu PJ, Srivastav A, Amaya A, et al. Association of provider recommendation and offer and influenza vaccination among adults aged ≥18 years - United States. Vaccine. 2018;36(6):890-898. doi:10.1016/j.vaccine.2017.12.016 3. Centers for Disease Control and Prevention. Preliminary estimated flu disease burden 2024-2025 flu season. Accessed July 23, 2025. https://www.cdc.gov/flu-burden/php/data-vis/2024-2025.html 4. Centers for Disease Control and Prevention. Influenza vaccine coverage, adults (reviewed May 7, 2025). Accessed June 24, 2025. https://www.cdc.gov/flu/fluvaxview/dashboard/vaccination-adult-coverage.html 5. Centers for Disease Control and Prevention. People at increased risk for flu complications. Accessed October 9, 2024. https://www.cdc.gov/flu/highrisk/index.htm 6. Centers for Disease Control and Prevention. Flu symptoms and complications. Accessed July 29, 2025. https://www.cdc.gov/flu/signs-symptoms/ 7. Centers for Disease Control and Prevention. Laboratory-confirmed influenza hospitalizations. Accessed July 18, 2025. https://gis.cdc.gov/grasp/fluview/FluHospChars.html 8. Marchi S, Fallani E, Salvatore M, et al. The burden of influenza and the role of influenza vaccination in adults aged 50-64 years: a summary of available evidence. Hum Vaccin Immunother. 2023;19(2):2257048. doi:10.1080/21645515.2023.2257048 9. American Heart Association. Complications from flu largely preventable with annual flu vaccine. Accessed July 1, 2025. https://newsroom.heart.org/news/complications-from-flu-largely-preventable-with-annual-flu-vaccine 10. Centers for Disease Control and Prevention. Flu vaccine safety and pregnancy. Accessed June 27, 2025. https://www.cdc.gov/flu/highrisk/qa_vacpregnant.htm 11. Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2023-24 influenza season. Accessed June 27, 2025. https://www.cdc.gov/fluvaxview/coverage-by-season/2023-2024.html#:~:text=An%20annual%20flu%20vaccine%20is,and%20unexpired%20vaccine%20is%20available 12. Centers for Disease Control and Prevention. National Center for Immunization and Respiratory Diseases. What to know about getting flu, COVID-19, and RSV vaccines at the same time. Accessed July 1, 2025. https://www.cdc.gov/ncird/whats-new/getting-vaccines-at-same-time.html 13. Centers for Disease Control and Prevention. Flu vaccine and people with egg allergies. Accessed June 30, 2025. https://www.cdc.gov/flu/prevent/egg-allergies.htm 14. Fluzone. Prescribing Information. Sanofi Pasteur Inc. 15. Flublok. Prescribing Information. Protein Sciences Corporation. 16. Fluzone High-Dose. Prescribing Information. Sanofi Pasteur Inc.
