Only ALTUVIIIO provided Factor VIII activity levels in the normal to near-normal (>40%) range for most of the week in the Phase 1 study of adults1
Phase 1 sequential PK study2
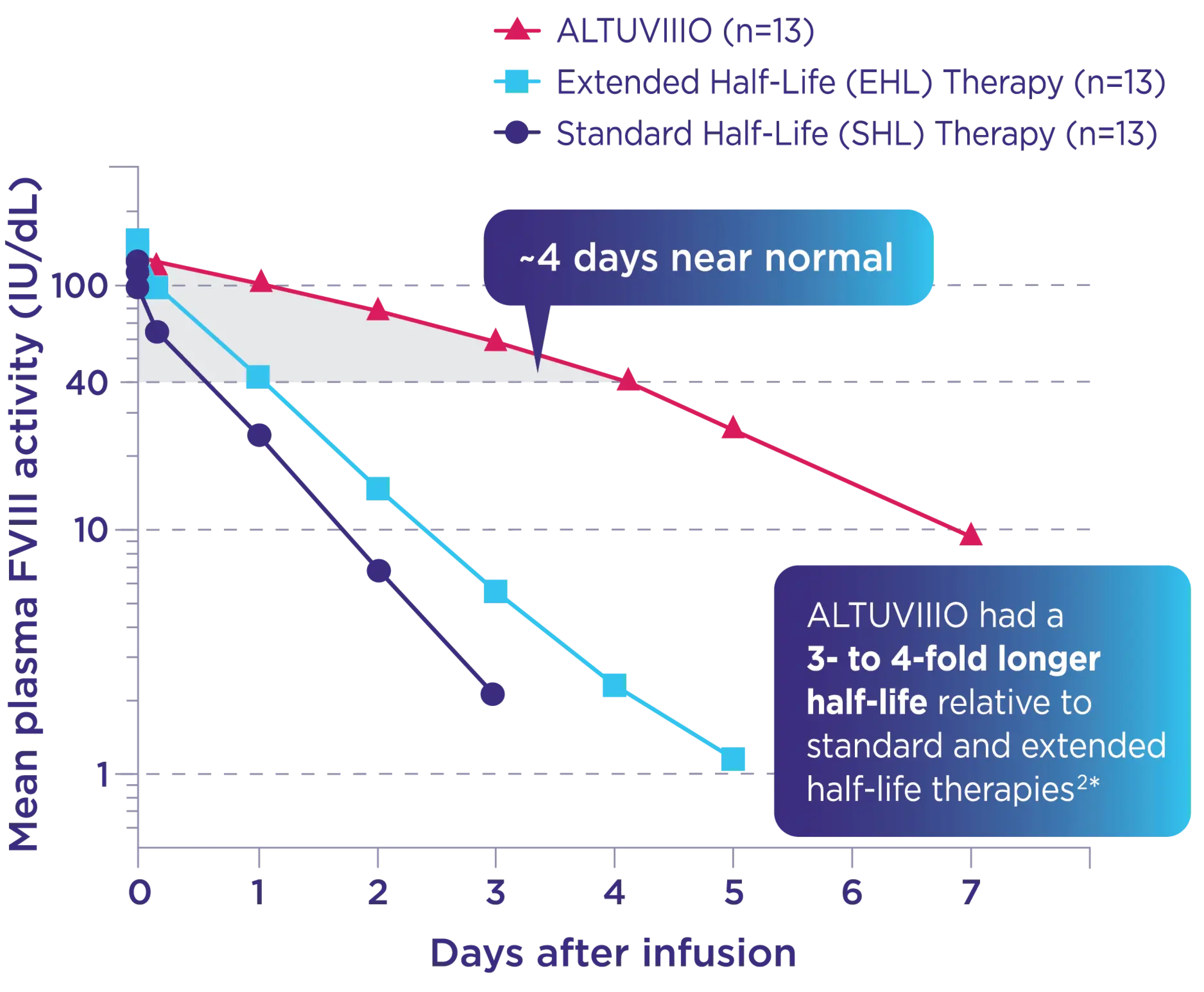
Lower interpatient variability vs an SHL and EHL* was observed in the Phase 1 study across PK parameters2†
* Data from a Phase 1 trial in 13 previously treated adults with severe hemophilia A. Pharmacokinetic profiles of ALTUVIIIO, Advate® [Antihemophilic Factor (Recombinant)], and Adynovate® [Antihemophilic Factor (Recombinant), PEGylated] were evaluated after sequential IV infusions. Primary objective: assess the elimination half-life for each product. Secondary objectives: characterization of additional PK parameters; evaluation of safety and tolerability of ALTUVIIIO.3
† Lower interpatient variability assessed across PK parameters (AUC, half-life, clearance) was also seen in XTEND-1.4
Advate and Adynovate are registered trademarks of Baxalta Incorporated, a Takeda company.
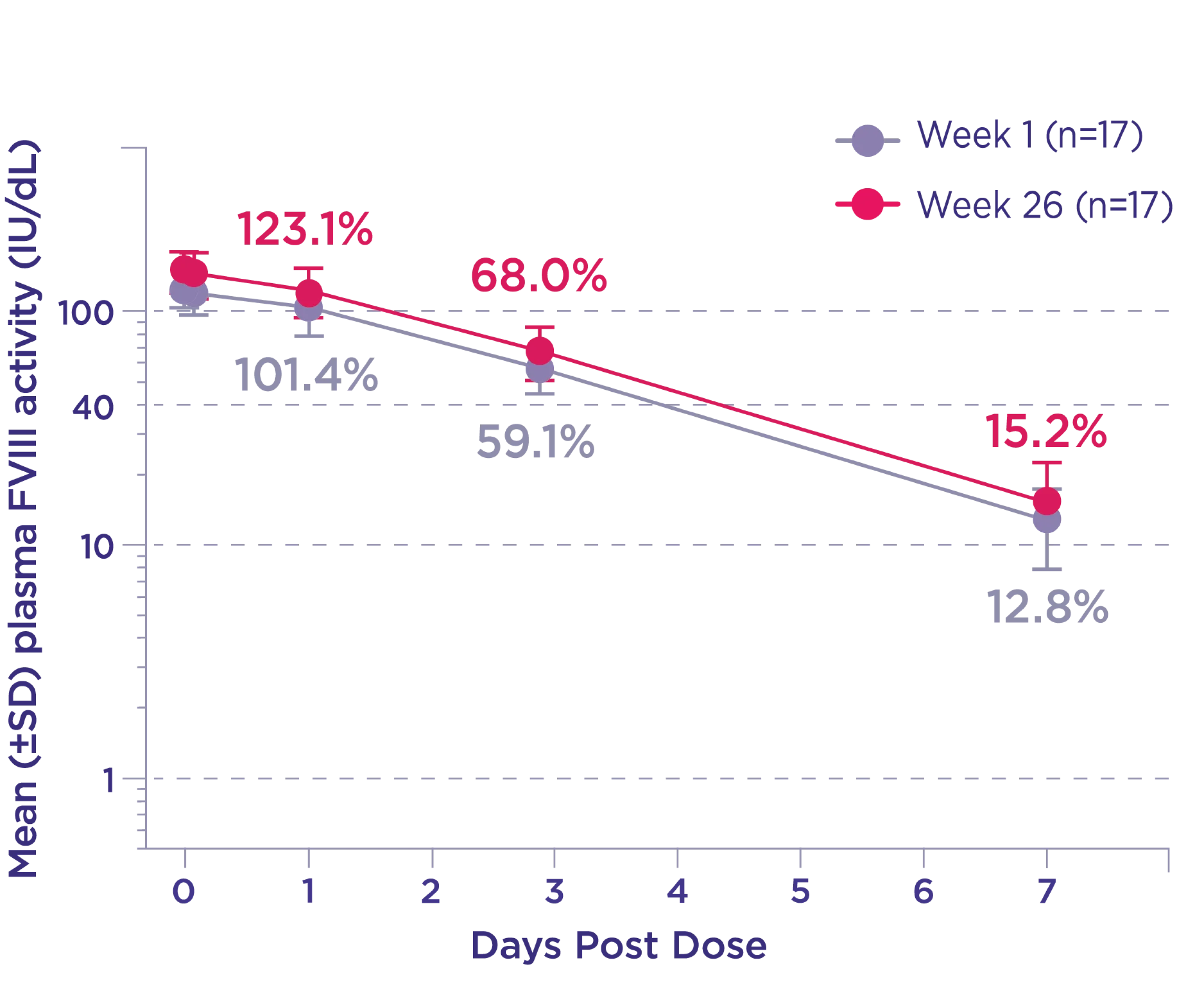
Normal to near-normal Factor VIII activity levels for most of the week were also seen in the Phase 3 XTEND-1 trial4*
Phase 3 XTEND-1 sequential PK subgroup (n=17)4
XTEND-1 trial adult study population (n=125)1
Once-weekly ALTUVIIIO provided
- ~4 days with mean Factor VIII levels above 40% (normal to near-normal range)1
- Factor VIII trough levels of 18.0%1†
- Factor VIII clearance has been demonstrated to be higher in patients <18 years, resulting in lower trough levels1
The pharmacokinetic profile was similar after the first dose and after 26 weeks of weekly dosing. A steady state was achieved after the first dose of ALTUVIIIO.1
Once-weekly 50 IU/kg ALTUVIIIO provided Factor VIII activity levels that were associated with a low bleed risk5
* Data from the Phase 3 XTEND-1 study in 134 adults (≥18 years) receiving a weekly IV infusion of 50 IU/kg ALTUVIIIO. PK parameters shown are based on plasma Factor VIII activity measured by the aPTT-based one-stage clotting assay.1
† Mean trough level was computed using available measurements at Week 52/end of study PK sampling visit for the adult population in the XTEND-1 study (n=123).1
Indication
aPTT=activated partial thromboplastin time; AUC=area under the curve; FVIII=Factor VIII; IU=international unit; IV=intravenous; PK=pharmacokinetic; rFVIII=recombinant Factor VIII; SD=standard deviation.
References: 1. ALTUVIIIO Prescribing Information. Bioverativ Therapeutics Inc. Waltham, MA. 2. Lissitchkov T, et al. Res Pract Thromb Haemost. 2023;7(4):100176. 3. Lissitchkov T, et al. Haemophilia. 2022;1227074. 4. von Drygalski A, et al. N Engl J Med. 2023;388(4):310-318.