- Article
- Source: Campus Sanofi
- Jul 9, 2025
Rezurock Efficacy
_430x268.jpg)
Rezurock demonstrates strong efficacy with durable ORR across all organs and FFS, along with meaningful improvements in various endpoints and QoL, as supported by clinical trials and RWE across diverse markets.1
.png)
Clinical Trial (ROCKstar* 36-month follow-up)1-3

- 74%: overall response rate (ORR) at 36 months
- Subgroups Best ORR
- 75%: Severe cGVHD patients
- 72%: Prior ibrutinib cGVHD patients
- 69%: Prior ruxolitinib cGVHD patients
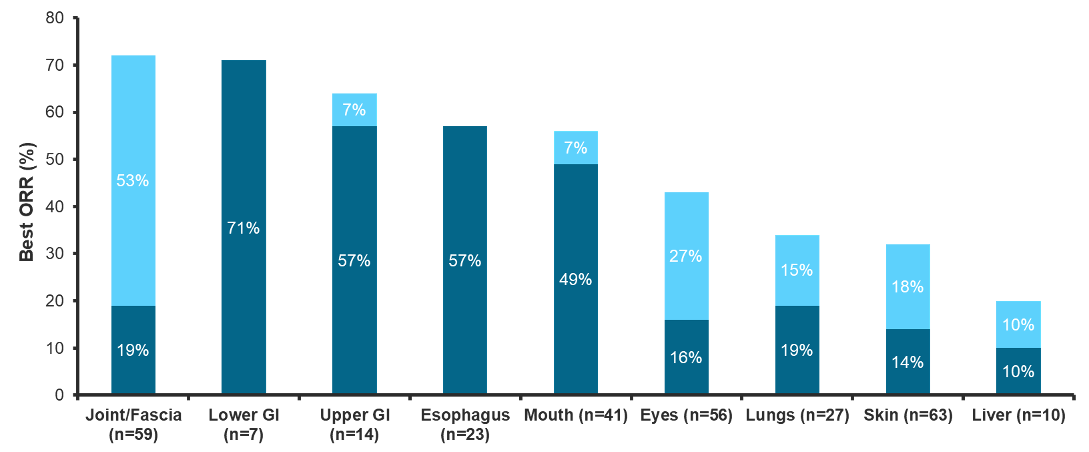
- Organ-specific complete response:
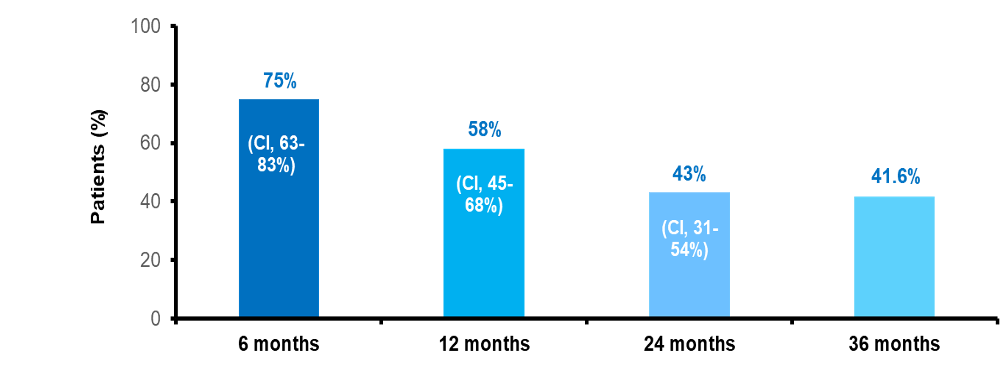
- Failure free survival (FFS)
- 86%: Overall survival (OS) at 24months

- 69.9 weeks/16 months: Median duration of response (DoR)

- 68% of the responders experienced clinically meaningful improvement in the Lee Symptom Scale (LSS)
*All the results are presented for the 200 mg once daily dosage (N=77)
RWE
- 44.2% improvement in ORR at 6 months (ref 4): (space) 39% of patients receiving Rezurock (LOT- episodes = 113) achieved a response (space) 27% of those receiving BAT (LOT-episodes = 245) achieved a response
- 28% improvement in FFS at 1 year(ref 4): 61.2% of patients treated with Rezurock (LOT-episodes = 113) remained failure-free 47.8% of those on BAT (LOT-episodes = 245) remained failure-free
Belumosudil showcased promising efficacy and safety results with no new safety signals identified. This was consistently demonstrated across various country cohorts:
France: In patients receiving at least two prior lines of therapy (N=68).
Germany and Switzerland: In heavily pretreated patients (N=33).6
Canada: In patients with a history of extensive treatment (N=35).
Spain: In patients receiving at least two prior lines of therapy (N=63).9
Abbreviations
ORR: Overall response rate; FFS: Failure-free survival; QoL: Quality of life; RWE: Real-world evidence; GI : Gastrointestinal; n: Sample size; DoR: Duration of response; LOT: Line of therapy; BAT: Best available therapies; cGVHD: Chronic graft-versus-host disease.
-
Data on Sanofi file, mini-CVD, Final Draft_without naïve comparison section_clean, Phase 2 ROCKstar data cutoff September 2022.
-
Lee, Stephanie J., et al. "Belumosudil for Chronic Graft-Versus-Host Disease after 2 or More Prior Lines of Systemic Therapy: 3-Year Follow-up of the Rockstar Study." Transplantation and Cellular Therapy, Official Publication of the American Society for Transplantation and Cellular Therapy 30.2 (2024): S262-S263.
-
Rezurock IVA – DRAFT – Will be on promomat
-
Data on Sanofi file, RockReal CSR, July 18 2024 / EBMT 2025 presentation Efficacy and Safety of Belumosudil as Compared with Best Available Therapy for the Treatment of cGVHD in the US - Kevin Hall1*, Dr. Aleksandr Lazaryan2*, Mark van der Laan3,4, Catherine J. Lee5, Aaron C. Logan6, Susan Gruber3, Shaum Kabadi7, Irfan Khan7, Charlie Nicholls8, Lauren Rota7, Enkeleida Nikai9, Ekaterina Ponomareva10, Alexandra Koumas10, Edmund K. Waller1
-
AAC Rezurock – Résumé du rapport périodique n° 2 – Période du 26/03/2024 au 25/09/2024 (17/01/2025). Available at: https://ansm.sante.fr/tableau-acces-derogatoire/rezurock
-
Heidenreich, Silke, et al. "Safety and efficacy of the ROCK-2-inhibitor Belumosudil in cGvHD treatment-a retrospective, German-Swiss multicenter real-world data analysis." Bone Marrow Transplantation (2025): 1-8. https://www.nature.com/articles/s41409-024-02507-9
-
Nihar Desai, etc. Real-World Experience of Belumosudil Treatment for Chronic Graft-Versus-Host Disease after the Failure of Multiple Lines of Therapy: a Canadian Experience. 2024 Annual Congress of the European Hematology Association (EHA). Poster. https://library.ehaweb.org/eha/2024/eha2024-congress/419429/nihar.desai.real-world.experience.of.belumosudil.treatment.for.chronic.html
-
Varon, Ben, et al. "Belumosudil Is Efficacious for the Management of Heavily Pretreated and Prolonged Chronic Graft-Versus-Host Disease: A Retrospective Multi-Center Real-World Study By the Israel Association for Bone Marrow Transplantation and Cellular Therapy." Blood 144 (2024): 7338. https://www.sciencedirect.com/science/article/pii/S0006497124101632
-
Efficacy and safety of belumosudil for treatment of cGVHD: multicenter retrospective analysis of the French cohort of the compassionate use program, on behalf of the French Society of Bone Marrow Transplantation and Cellular Therapy | Bone Marrow Transplantation https://www.nature.com/articles/s41409-025-02554-w
-
EBMT 2025 poster – B032
MAT-KW-2500349