- Article
- Source: Campus Sanofi
- 15 May 2025
Efficacy & Safety Profile
Beyfortus showed efficacy against RSV disease in infants1-3
Incidence of MA RSV LRTI (inclusive of hospitalisations) through 150 days post injection
(primary endpoint was met)1–3
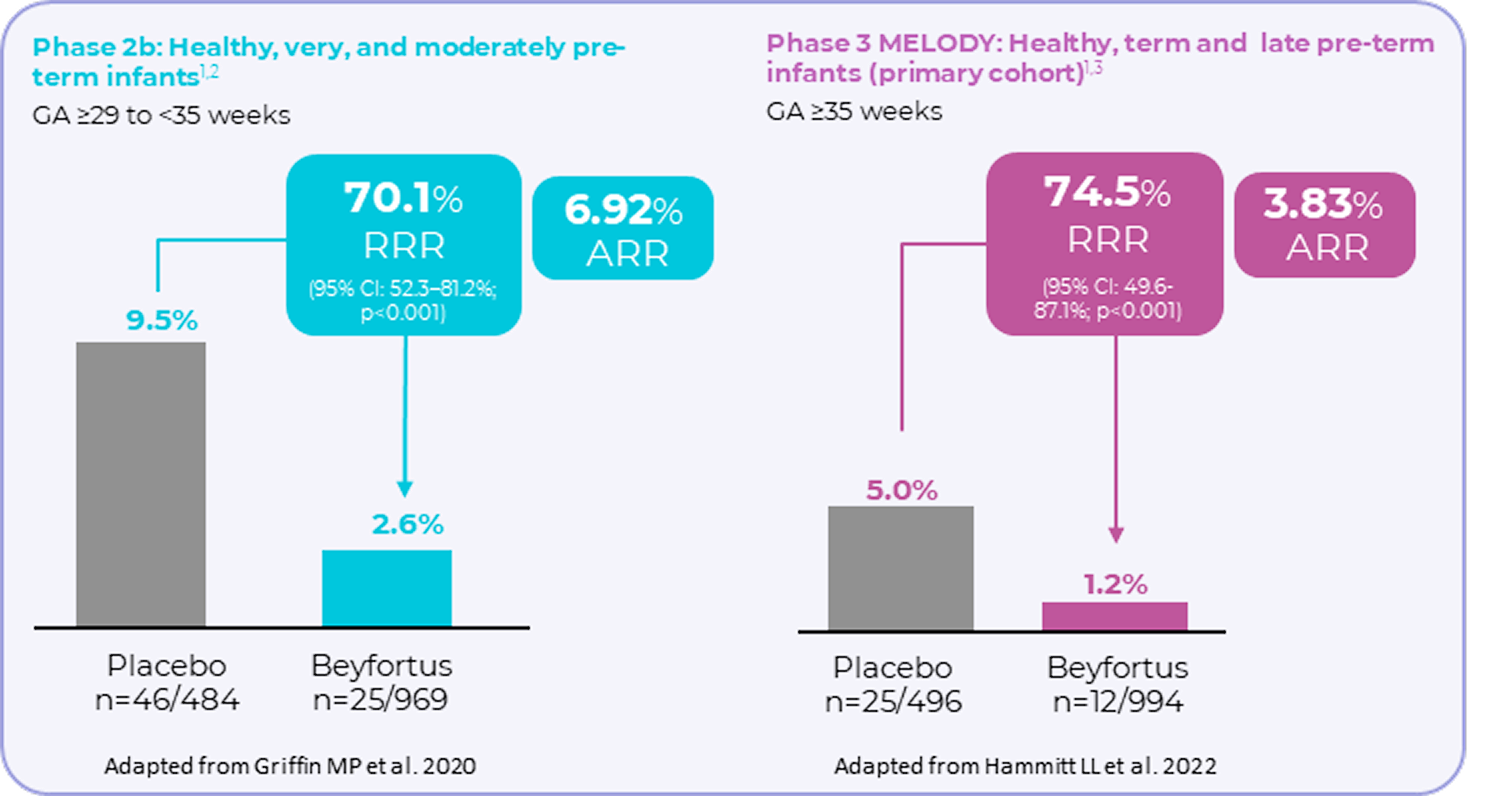
Phase 2b and Phase 3 pivotal clinical trials were randomised, double-blind, controlled trials.2,3 Phase 2b n= 1,453 and phase 3 n=1,490. Efficacy for MA RSV-LRTI based on relative risk reduction against placebo was adjusted for age at randomisation and hemisphere. Medically attended (MA) includes all healthcare provider visits such as physician’s office, urgent care, emergency room, and hospitalisations.
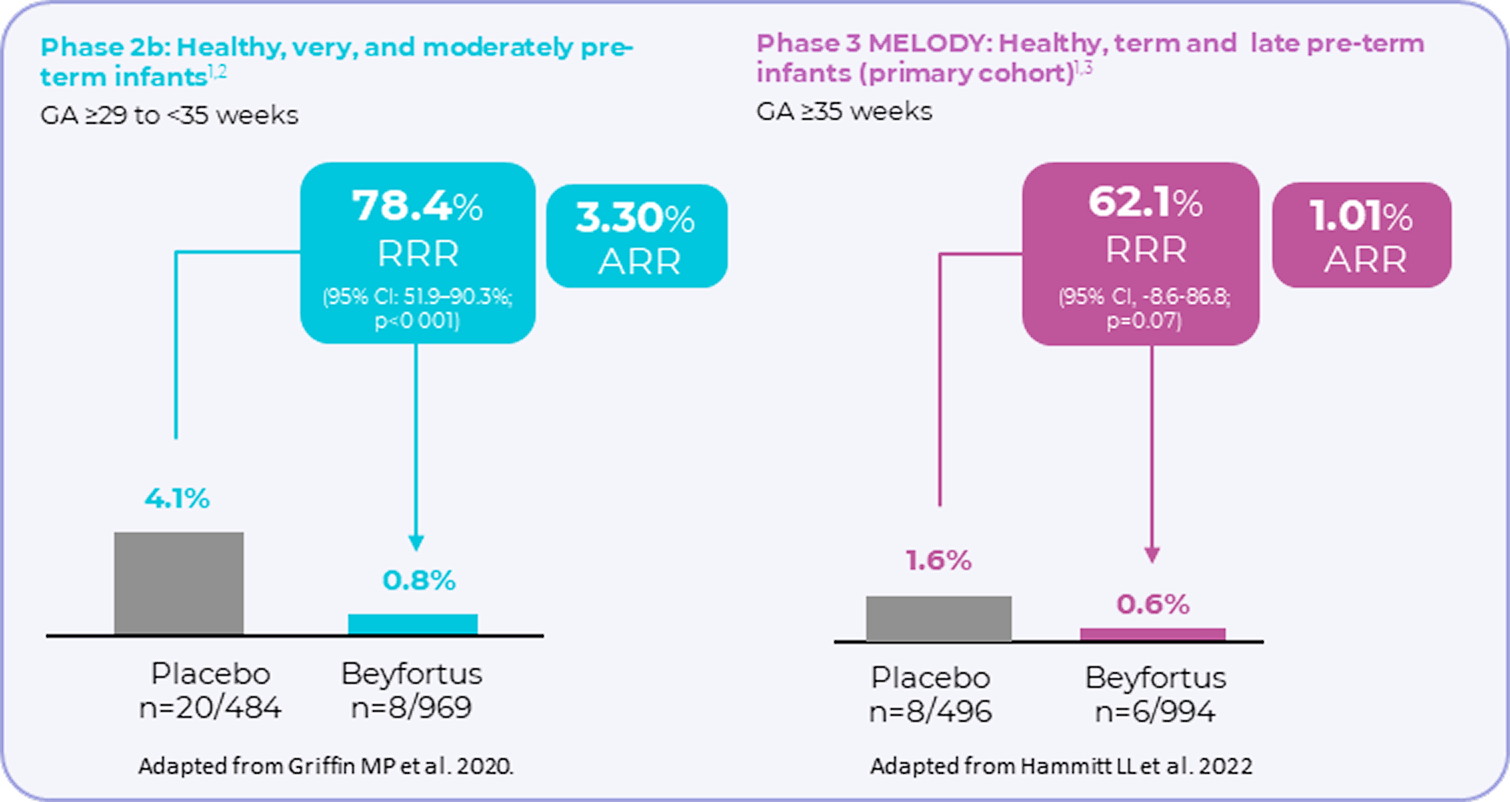
Beyfortus demonstrated a reduction in RSV hospitalisation1-3
Incidence of hospitalisation for RSV-related LRTI (secondary endpoint) in pivotal clinical
trials1–3
The incidence of RSV hospitalisation showed consistent results in a close to real-world settings*4
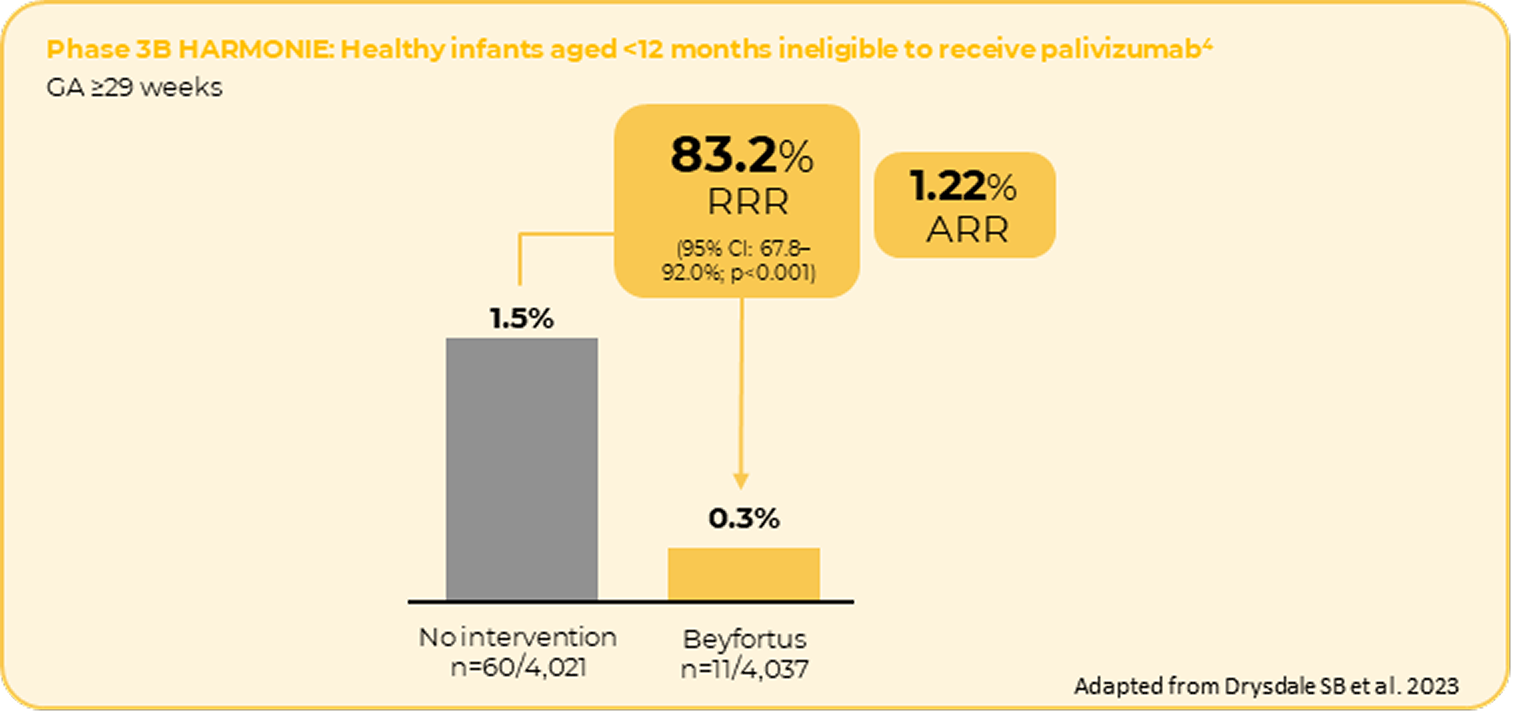
* The Hospitalised RSV Monoclonal Antibody Prevention (HARMONIE) study is a large, multi-country European interventional clinical trial aiming to determine the efficacy and safety of a single intramuscular dose of nirsevimab, with data collected in a real-world setting during the 2022-2023 RSV season. The trial recruited more than 8,000 infants and took place at nearly 250 sites across France, Germany and the United Kingdom.
Consistent safety profile across multiple infant cohorts
Safety has been studied in a broad population, including healthy pre-term/term babies and those at higher risk of RSV-related LRTI.1-3,5
The safety profile was similar to placebo in the phase 2b and phase 3 studies.2-3,5
The most frequent adverse reaction was rash (0.7%) occurring within 14 days post dose. Most cases were mild to moderate in intensity. Additionally, pyrexia and injection site reactions were reported at a rate of 0.5% and 0.3% within 7 days post dose, respectively.
Injection site reactions were non-serious.1
Infants at higher risk for severe RSV
The safety profile was comparable to palivizumab in first RSV season and consistent with the safety profile in term and preterm infants (≥29 GA weeks).1,5,a
Infants who remain vulnerable to severe RSV in their second season
The safety profile was consistent with the safety profile observed during their first RSV season.1
For the full list of adverse events please refer to the Beyfortus SmPC.

AAR, absolute risk reduction; CI, confidence internal; LRTI, lower respiratory tract infection; RRR, relative risk reduction; RSV, respiratory syncytial virus; GA, gestational age
a. Phase 2/3 MEDLEY study with n=925 infants born very or moderately pre-term, or infants with congenital heart disease or chronic lung disease.
- Beyfortus. Summary of Product Characteristics.
- Griffin MP et al. N Engl J Med 2020; 383(5): 415-425 & Supplementary Appendix.
- Hammitt LL et al. N Engl J Med 2022; 386(9): 837-846 & Supplementary Appendix.
- Drysdale SB et al. N Engl J Med 2023; 389(26): 2425–2435 & Protocol.
- Domachowske J et al. N Engl J Med 2022; 386(9): 892-894.
MAT-XU-2501617 (v2.0) Date of preparation: September 2025
