
Beyfortus has the power to help reduce the chaos of the RSV season
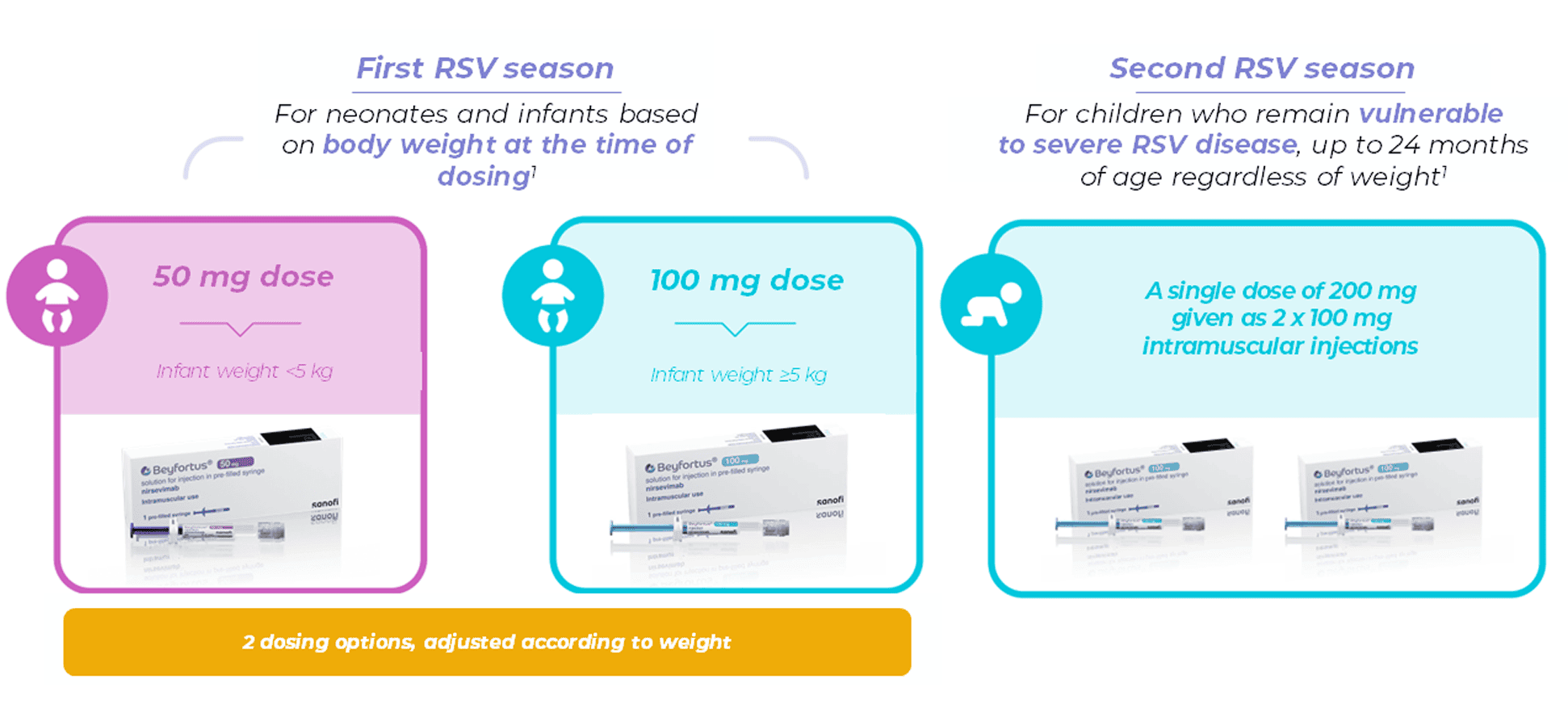
Beyfortus is indicated for the prevention of Respiratory Syncytial Virus (RSV) lower respiratory tract disease in:1
i. Neonates and infants during their first RSV season
ii. Children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season
Beyfortus should be used in accordance with official recommendations.1
Beyfortus is licensed in the UK for a broad range of infant populations entering their first and second RSV season, including those born:1

Healthy or With Underlying Conditions*

Full Term or Preterm*

Before or During the RSV Season*
*Underlying conditions include high risk group of infants including extremely preterm infants (GA <29 weeks) entering their first RSV season, children with chronic lung disease of prematurity or haemodynamically significant congenital heart disease ≤24 months of age entering their first or second RSV season and immunocompromised infants and children ≤24 months. There are limited data available in extremely preterm infants (Gestational Age [GA] <29 weeks) less than 8 weeks of age.

Beyfortus is the first RSV immunisation designed to offer protection to all infants,
with effectiveness and public health impact supported by real world data1,2-6
Approximately 6 million infants have been immunised with Beyfortus worldwide**,7
**Supported by global estimated exposure based on calculation from sales volume data (01 September 2023 through 31 October 2024). The number of children exposed is estimated based on distribution of recommended dose of Beyfortus administered, considering body weight, season of administration (1st or 2nd) and immunisation plan.

Direct
Offers direct protection against RSV LRTD via administration of antibodies1 without relying on an infant’s immune system8
Rapid
Offers RSV protective antibodies starting day 1 after injection†,1,9
Durable
Beyfortus is a long-acting monoclonal antibody modified to extend the half-life to ~71 days‡,1
A single dose offers protection for at least 5 to 6 months1
Beyfortus is a recombinant neutralising human monoclonal antibody that provides long-acting passive immunity to RSV.
It binds to a conserved epitope on the RSV prefusion F protein, interfering with the membrane fusion step in the viral entry process, neutralising the virus and blocking cell-to-cell fusion1
Beyfortus should be administered as a single dose for infants born during the RSV season or prior for those born outside the season1
It is available in 2 pre-filled syringes based on the infant’s weight at the time of receiving Beyfortus.1
Beyfortus is given as an intramuscular injection, preferably in the anterolateral aspect of the thigh.1

Direct
Offers direct protection against RSV LRTD via administration of antibodies1 without relying on an infant’s immune system8
Rapid
Offers RSV protective antibodies starting day 1 after injection†,1,9
Durable
Beyfortus is a long-acting monoclonal antibody modified to extend the half-life to ~71 days‡,1
A single dose offers protection for at least 5 to 6 months1
Beyfortus is a recombinant neutralising human monoclonal antibody that provides long-acting passive immunity to RSV.
It binds to a conserved epitope on the RSV prefusion F protein, interfering with the membrane fusion step in the viral entry process, neutralising the virus and blocking cell-to-cell fusion1
Beyfortus should be administered as a single dose for infants born during the RSV season or prior for those born outside the season1
It is available in 2 pre-filled syringes based on the infant’s weight at the time of receiving Beyfortus.1
Beyfortus is given as an intramuscular injection, preferably in the anterolateral aspect of the thigh.1

†Following IM administration, maximum concentration was reached within 6 days (range 1–28 days).1
‡Beyfortus has been modified with a triple amino acid substitution (YTE) in the Fc region to extend serum half-life.1
Beyfortus is supported by comprehensive evidence, reinforced by real-world studies1, 2-6,10-12
Resources
Get ready for the RSV season: Watch educational & training videos for Beyfortus.
Use the following downloadable resources to support with the administration of Beyfortus.
Order printed materials
Request red book inserts for the child's immunisation record (Red Book) and parent cards with access to the parent information leaflet.
This can support parents whose child has received or will receive Beyfortus and the prescribing decision has been made by the healthcare professional.
Materials are produced and funded by Sanofi and can only be ordered by a registered healthcare professional in the UK.
Storage

How Beyfortus should be stored?1
- Store in a refrigerator (2°C ‑ 8°C).
- Do not freeze.
- Do not shake or expose to direct heat.
- Keep the pre‑filled syringe in the outer carton in order to protect from light.

Shelf life?1
- 3 years
- Beyfortus may be kept at room temperature (20°C ‑ 25°C) when protected from light for a maximum of 8 hours. After this time, the syringe must be discarded.

Pack size available?1
1 pre-filled syringe without needle
Safety profile summary
The most frequent adverse reaction was rash (0.7%) occurring within 14 days post dose. The majority of cases were mild to moderate in intensity. Additionally, pyrexia and injection site reactions were reported at a rate of 0.5% and 0.3% within 7 days post dose, respectively. Injection site reactions were non-serious. Please see SmPC for full list of adverse events.

LRTI, lower respiratory tract infection; RSV,Respiratory Syncytial Virus
- Beyfortus. Summary of Product Characteristics.
- Sumsuzzman D et al. Real-world effectiveness of nirsevimab against respiratory syncytial virus disease in infants: a systematic review and meta-analysis. SSRN. Available at: https://ssrn.com/abstract=5096762.
- Mallah N et al. Lancet Infect Dis 2024; 25(2): E62–E63.
- Lassoued Y et al. Lancet Reg Health Eur 2024; 44: 101007.
- Hsiao A et al. Ann Allergy Asthma Immunol 2024; 133(6) Supplement 2: S3–S4.
- NirseCL. Monitoring the impact of Nirsevimab in the winter campaign in 2024 in Chile – fifth report. Available at: https://nirse.isci.cl/#reporte. Accessed: June 2025.
- Sanofi Data on File. 2025.
- Verwey C and Madhi SA. BioDrugs 2023; 37: 295–309.
- Clegg L et al. J Clin Pharmacol 2024; 64(5): 555–567.
- Domachowske JB et al. N Engl J Med 2022; 386(9): 892–894 & Supplementary Appendix.
- Hammitt LL et al. N Engl J Med. 2022; 386(9): 837–846 & Supplementary Appendix.
- Drysdale SB et al. N Engl J Med 2023; 389(26): 2425-2435 & Supplementary Appendix.
MAT-XU-2501125 (v4.0) Date of preparation: October 2025



