
DUPXIENT is indicated for the treatment of adults with moderate-to-severe prurigo nodularis (PN) who are candidates for systemic therapy.
Prurigo nodularis – much more than a skin condition1–4

Prurigo nodularis (PN) is a distinct condition, not simply a subset of atopic dermatitis (AD).5 The chronic nature of PN is debilitating, with patients experiencing itch that is among the most severe in chronic itch conditions.1 2 in 3 patients (59/88; 67%) cite chronic itch in both nodules and inter-lesional skin*1 - and these painful, burning nodules can often bleed from repeated scratching, resulting in erosion and hyperpigmentation.6,7
Beyond the physical symptoms of PN, patients also experience a reduced quality of life (QoL),1,6,8 with the majority (24/39; 61.5%) reporting that pruritus disturbed their sleep to a great extent✝2 and almost half (78/171; 45.6%) having diagnosed anxiety.*1
An alternative approach to PN is needed
Patients with PN require a novel approach to help improve itch, nodules, and QoL.8,9 Only around a quarter of patients (13/49; 26.5%) report a positive response to one of the main PN treatments, topical steroids.‡9Additionally, among patients with PN, there are low levels of satisfaction with available therapies,8 with almost 60% of patients (225/396; 56.8%) reporting that they were dissatisfied with the treatments they had received in the previous 6 months.5,8
PN can impact several aspects of a patient’s life,1–4 therefore, it is important to identify people who are in need of a different treatment option.
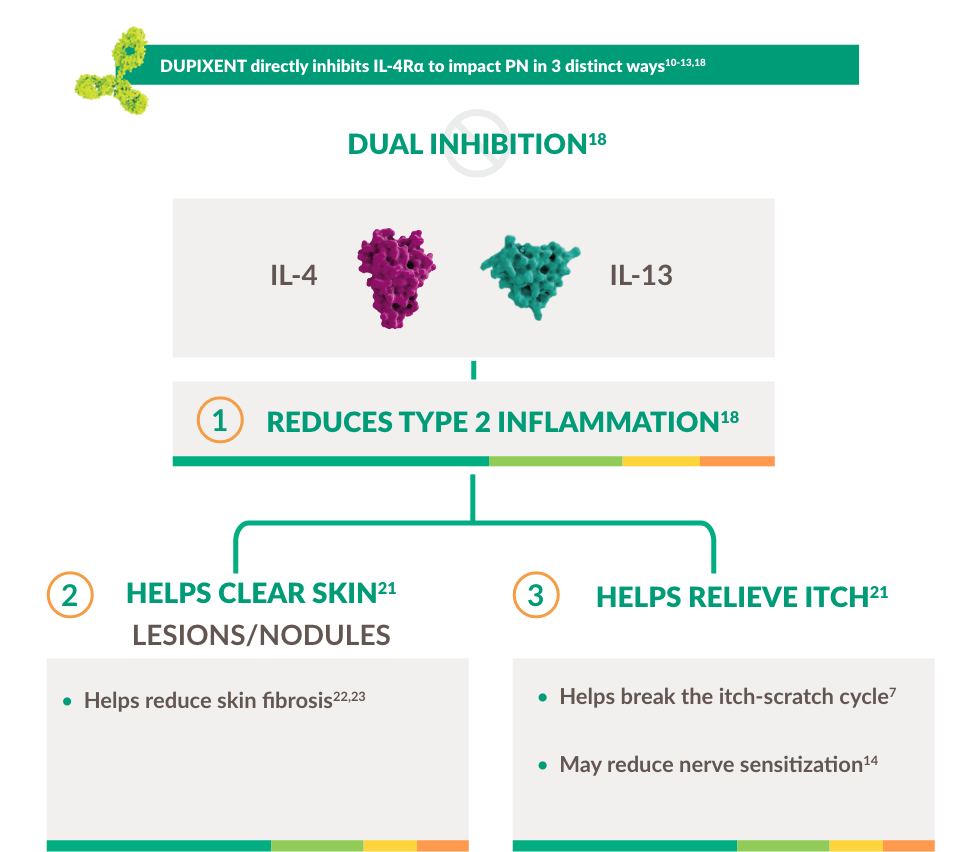
Prurigo nodularis - driven by Type 2 inflammation10-13

Type 2 inflammation is a key driver of PN, with IL-4 and IL-13 in particular having direct and indirect effects on itch.10–15 Increased IL-4 and ll -13 signaling leads to increased activity of inflammatory mediators such as Th2, Th17 and Th22 cells, as well as eosinophils and basophils.10–13 This in turn can lead to a chronic itch-scratch cycle that leads to nodules, fibrosis and scarring.13,15–17
Intense itch and chronic inflammation lead to disfiguring nodules and hyperpigmentation7,10-13
DUPIXENT is the first and only targeted immunomodulator to specifically inhibit IL-4 and IL-13 signaling key drivers of persistent underlying Type 2 inflammation18–20

DUPIXENT offers significant improvements in symptoms and QoL vs placebo21

PRIME study design:21

PRIME was a randomised, phase 3, double-blind, placebo-controlled trial that evaluated the efficacy and safety of DUPIXENT in 151 adults with PN inadequately controlled with topical prescription therapies or for whom those therapies were not advisable. During the 24-week treatment period, subjects received DUPIXENT or placebo every 2 weeks with or without topical treatments (low- or medium-dose topical corticosteroids or topical calcineurin inhibitors were continued if subjects were using these treatments at randomisation).
The primary endpoint was the proportion of subjects with clinically meaningful improvement in itch at 24 weeks (measured by a ≥ 4-point reduction in WI-NRS* of 0–10) was met.
Footnotes:
*WI-NRS is a patient-reported outcome comprising 1 item, the worst itch over the past 24 hours, rated from 0 (no itch) to 10 (worst imaginable itch). In PN, a reduction of ≥ 4 in WI-NRS score is considered clinically meaningful for baseline scores between 7 and 9.24
PRIME2 study design:21

PRIME2 was a randomised, phase 3, double-blind, placebo-controlled trial that evaluated the efficacy and safety of DUPIXENT in 160 adults with PN inadequately controlled with topical prescription therapies or for whom those therapies were not advisable. During the 24-week treatment period, subjects received DUPIXENT or placebo every 2 weeks with or without topical treatments (low- or medium-dose topical corticosteroids or topical calcineurin inhibitors were continued if subjects were using these treatments at randomisation).
The primary endpoint was the proportion of subjects with clinically meaningful improvement in itch at 12 weeks (measured by a ≥4-point reduction in WI-NRS* of 0–10) was met.
Footnotes:
*WI-NRS is a patient-reported outcome comprising 1 item, the worst itch over the past 24 hours, rated from 0 (no itch) to 10 (worst imaginable itch). In PN, a reduction of ≥ 4 in WI-NRS score is considered clinically meaningful for baseline scores between 7 and 9.24
Itch

Significantly more patients achieved clinically meaningful improvements in itch intensity vs placebo at Week 24 (primary endpoint)21

- 60% (45/75) of patients on DUPIXENT achieved ≥4-point improvement in WI-NR5 at Week 24 vs 18.4% (14/76) with placebo (p<0.001)*21
- Nominally significant improvements (p<0.05) vs placebo in mean WI-NRS score were observed as early as Week 3 (secondary endpoint)*21
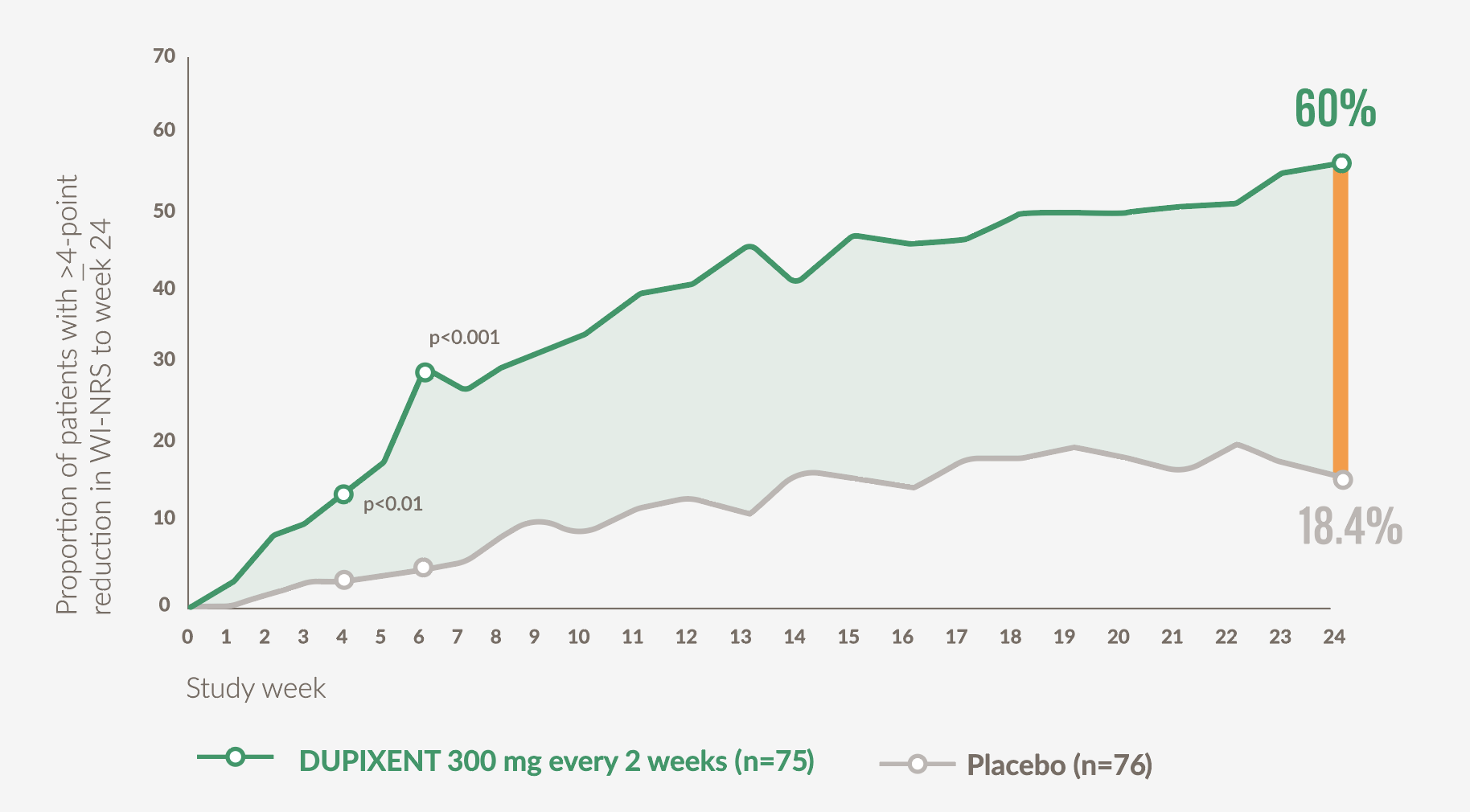
Graph adapted from Yosipovitch G. et al 202321
Footnotes:
*WI-NRS is a patient-reported outcome comprising 1 item, the worst itch over the past 24 hours, rated from 0 (no itch) to 10 (worst imaginable itch). In PN, a reduction of ≥4 in WI-NRS score is considered clinically meaningful for baseline scores between 7 and 9.24
Significantly more patients achieved clinically meaningful improvements in itch intensity vs placebo at Week 12 (primary endpoint), with effects sustained to Week 24 (secondary endpoint)21

- 37.2% (29/78) of patients on DUPIXENT achieved ≥4-point improvement in WI-NRS at Week 12 vs 22% (18/82) with placebo (p=0.02)*21
- 57.7% (45/78) of patients on DUPIXENT achieved ≥4-point improvement in WI-NRS at Week 24 vs 19.5% (16/82) with placebo (p<0.001)*21
- Nominally significant improvements (p<0.05) vs placebo in mean WI-NRS score were observed as early as Week 4 (secondary endpoint)*21
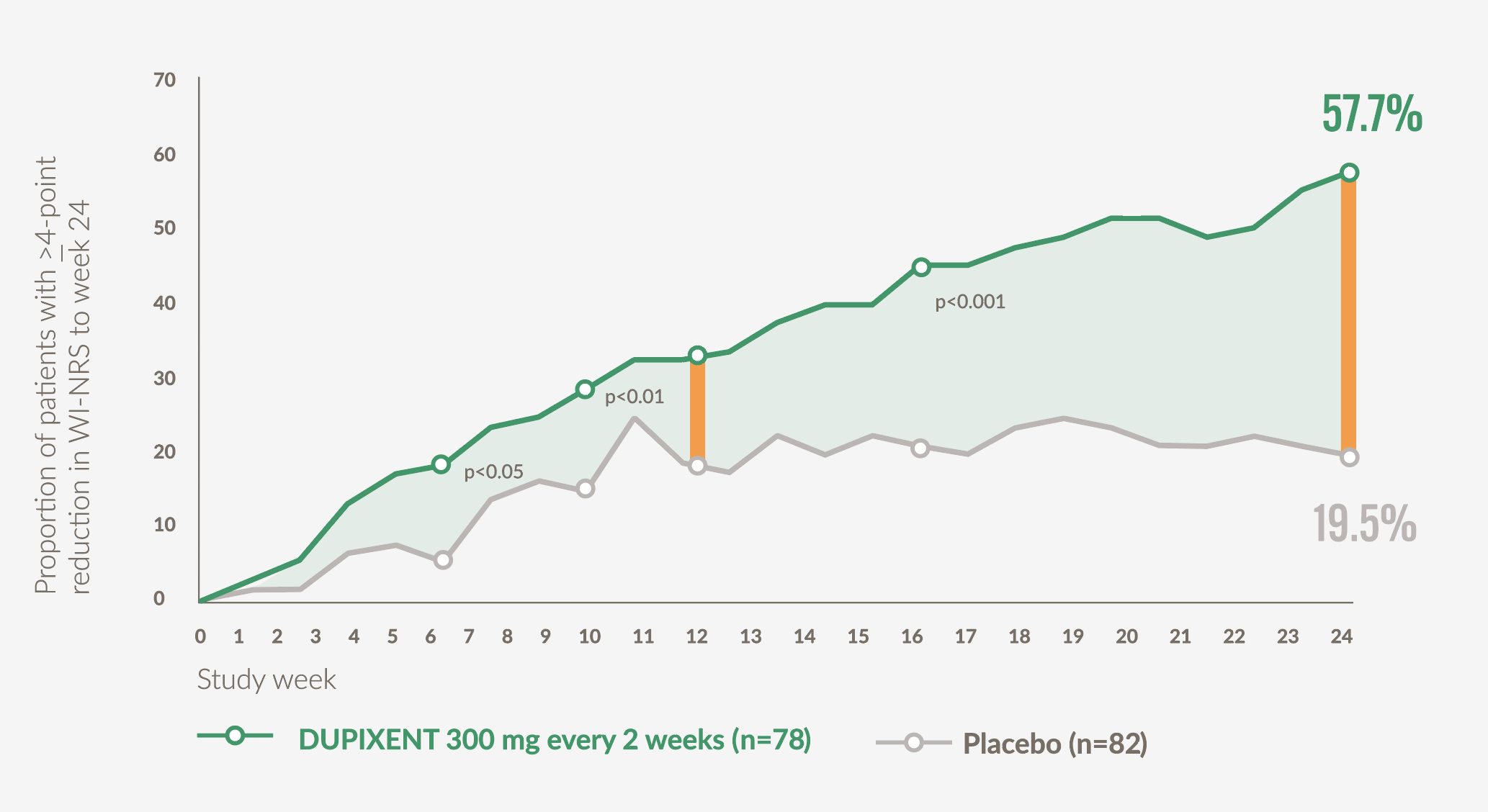
Graph adapted from Yosipovitch G. et al 202321
Footnotes:
*WI-NRS is a patient-reported outcome comprising 1 item, the worst itch over the past 24 hours, rated from 0 (no itch) to 10 (worst imaginable itch). In PN, a reduction of ≥4 in WI-NRS score is considered clinically meaningful for baseline scores between 7 and 9.24
Nodule Clearance

Significantly more patients achieved clear or almost clear skin with DUPIXENT vs placebo at Week 24 (secondary endpoint)21

- 48% (36/75) of patients on DUPIXENT achieved an IGA PN-S score of 0 or 1 at Week 24 vs 18.4% (14/76) with placebo (p<0.001)*21
- Significant improvements (p<0.05) vs placebo in proportion of patients with clear or almost clear skin were observed as early as Week 421
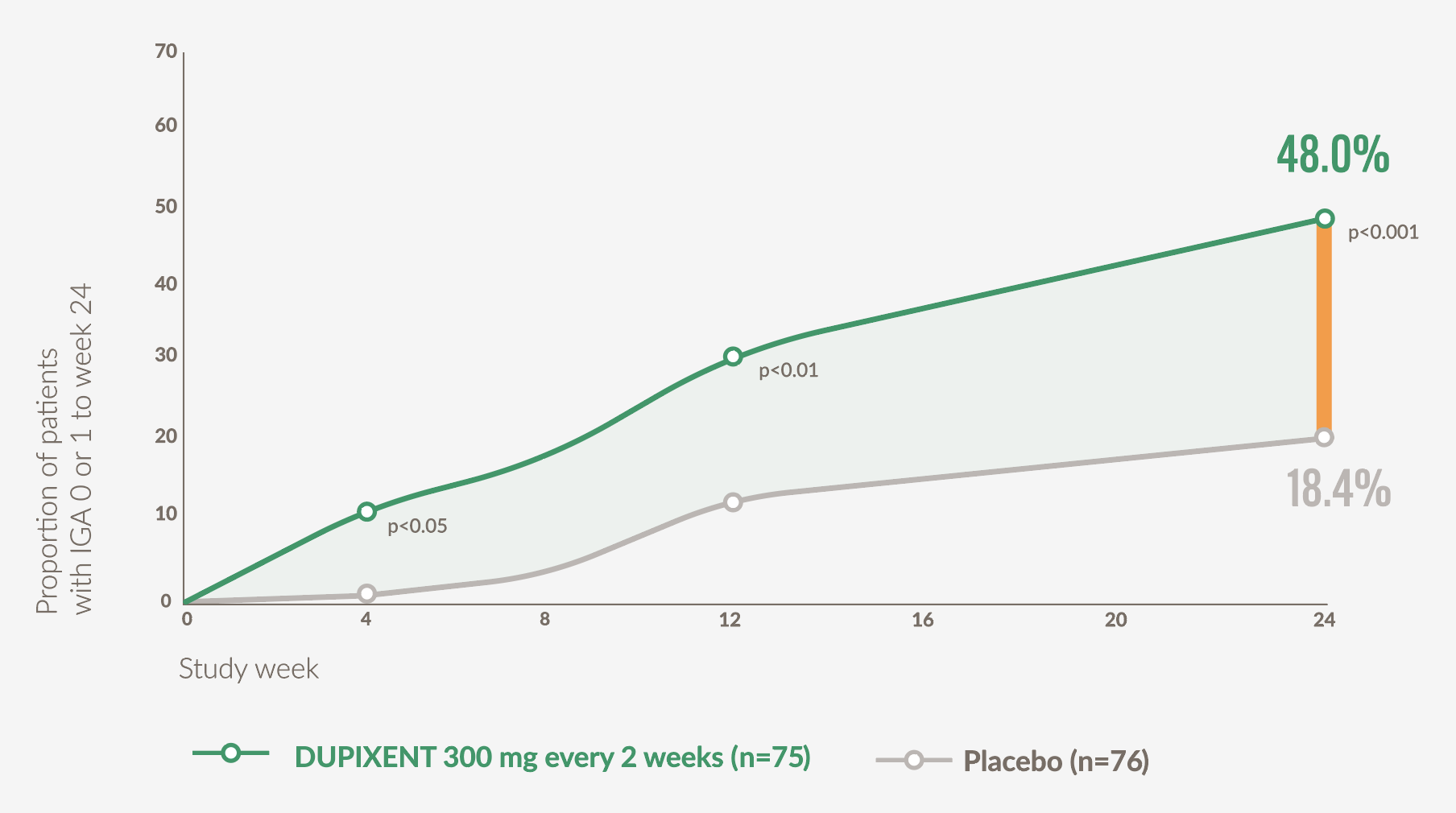
Graph adapted from Yosipovitch G. et al, 202321
Footnotes:
*IGA PN-S is a clinician-reported outcome that assesses disease stage based on the number of pruriginous lesions, rated from 0 (clear) to 4 (severe).24
Significantly more patients achieved clear or almost clear skin with DUPIXENT vs placebo at Week 24 (secondary endpoint)21

- 44.9% (35/78) of DUPIXENT patients achieved an IGA PN-S score of 0 or 1 at Week 24 vs 15.9% (13/82) with placebo (p<0.001)*21
- Significant improvements (p<0.05) vs placebo in proportion of patients with clear or almost clear skin were observed as early as Week 1221
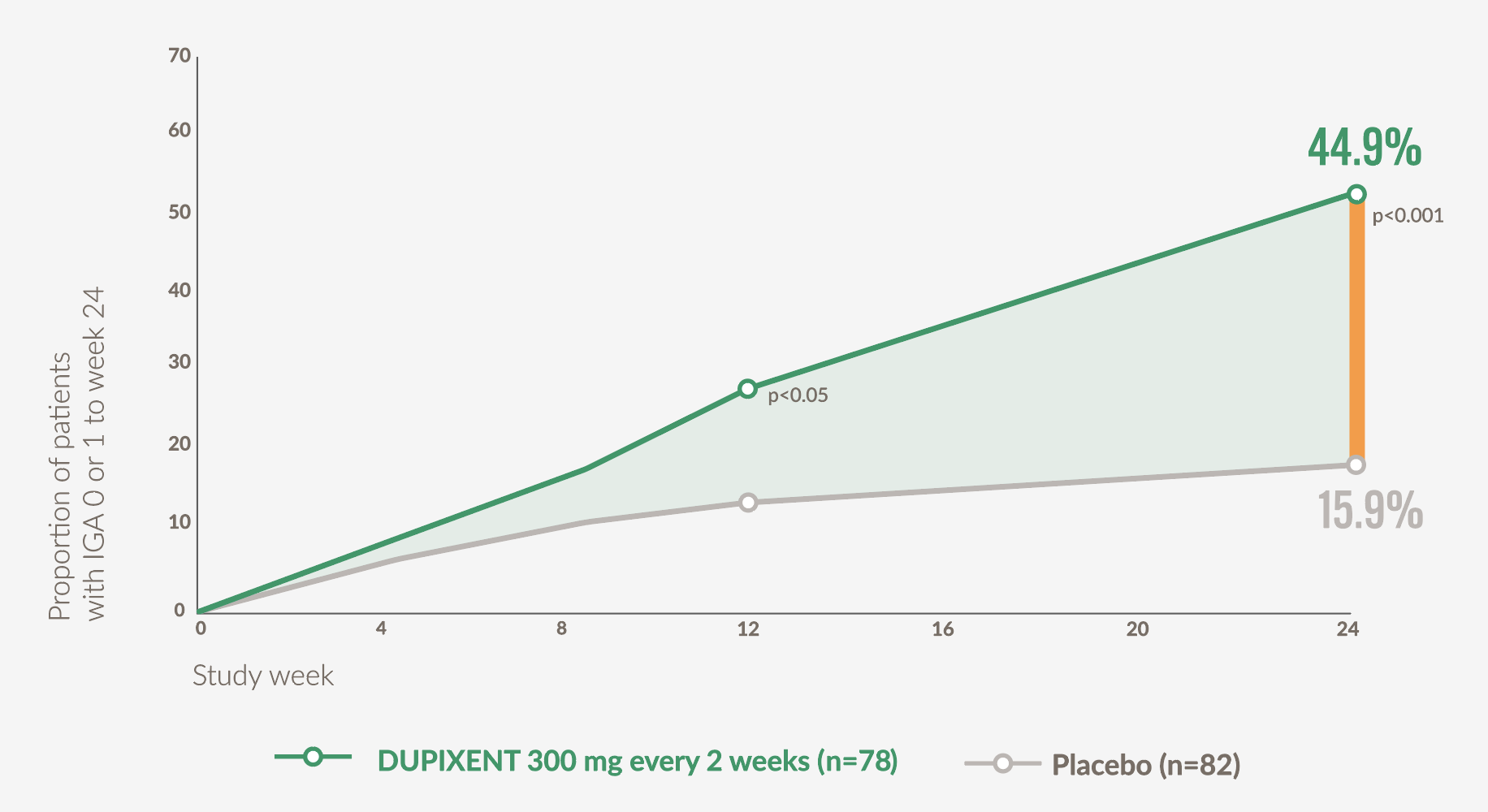
Graph adapted from Yosipovitch G. et al, 202321
Footnotes:
*IGA PN-S is a clinician-reported outcome that assesses disease stage based on the number of pruriginous lesions, rated from 0 (clear) to 4 (severe).24
Quality of Life (QOL)

DUPIXENT patients experienced significant improvements in QoL vs placebo at Week 24 (secondary endpoint)21

- Patients on DUPIXENT (n=75) achieved a mean 12.0-point (SE 1.0) improvement in DLQI vs 5.8 (SE 1.0) points with placebo (n=76) at Week 24 (p<0.001)*21
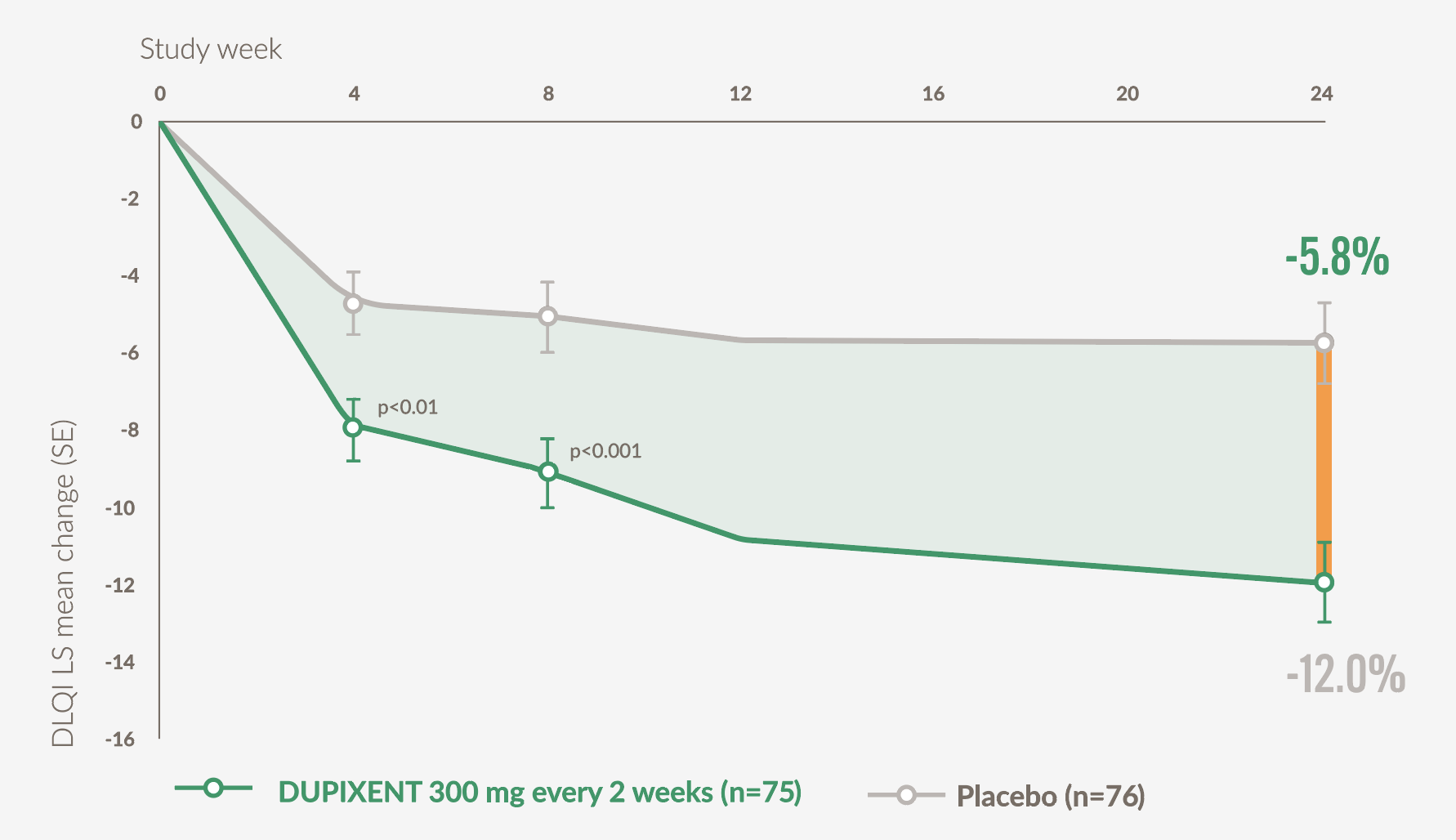
Graph adapted from Yosipovitch G. et al, 202321
Footnotes:
*DLQI score is based on a 10-item patient questionnaire that assesses 6 different aspects that may affect quality of life; symptoms and feelings, daily activities, leisure, work and school performance, personal relationships, and treatment. Each question is scored from 0 (not at all) to 3 (very much) and then totalled for the final score.25
DUPIXENT patients experienced significant improvements in QoL vs placebo at Week 24 (secondary endpoint)21

- Patients on DUPIXENT (n=78) achieved a mean 13.2-point (SE 1.2) improvement in DLQI vs 6.8 (SE 1.2) points with placebo (n=82) at Week 24 (p<0.001)*21
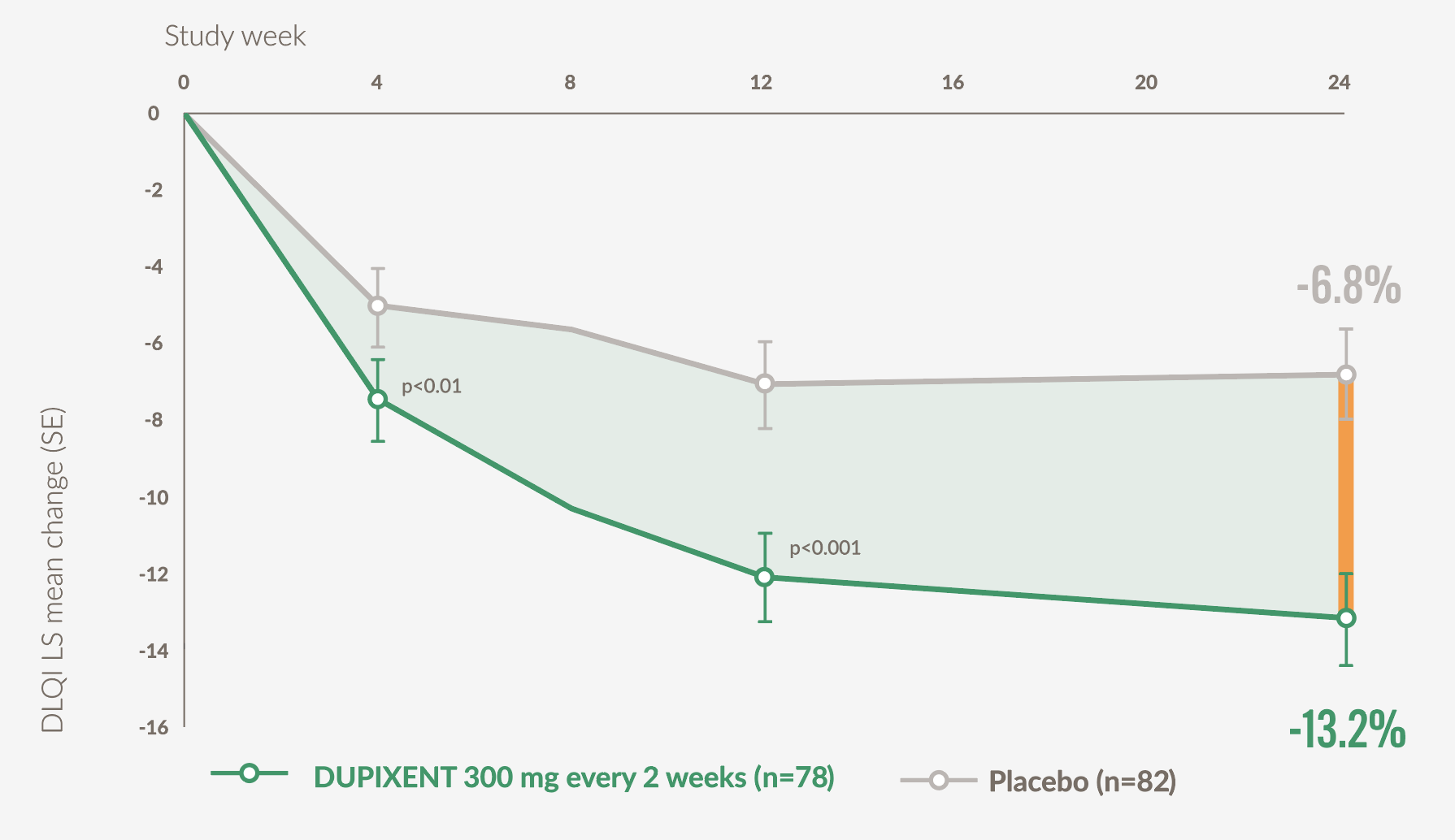
Graph adapted from Yosipovitch G. et al, 202321
Footnotes:
*DLQI score is based on a 10-item patient questionnaire that assesses 6 different aspects that may affect quality of life; symptoms and feelings, daily activities, leisure, work and school performance, personal relationships, and treatment. Each question is scored from 0 (not at all) to 3 (very much) and then totalled for the final score.25
Reimbursement
Scotland26
Scottish Medicines Consortium (SMC) advice
- Dupilumab is accepted for use for the treatment of adults with moderate-to-severe prurigo nodularis (PN) who are candidates for systemic therapy within NHS Scotland. For further guidance please follow this link SMC 2598.
England27
- Dupilumab is not recommended, within its marketing authorisation, for treating moderate to severe prurigo nodularis in adults when systemic treatment is suitable. For the full guidance please follow this link TA955.
Wales28
- Dupilumab is recommended as an option for use within NHS Wales in line the NICE TA955 guidance. For the full guidance please follow the link to the AWMSG advice.
Northern Ireland29
- Dupilumab is not accepted for use, within its marketing authorisation, for treating moderate to severe prurigo nodularis in adults when systemic treatment is suitable. For more information see NICE TA955.
Safety Profile
Safety Outcomes of PRIME and PRIME2 | ||
|---|---|---|
| TEAEs reported in ≥ 5% of patients in any treatment group (MedDRA PT), n (%) | ||
| TEAE groups of interest, n (%) | ||
| TEAE, n(%) | PRIME | PRIME 2 |
|
| Placebo (n = 82) DUPIXENT (n= 77) | |
| Patients with ≥ 1 TEAE | 42 (56.0) 49 (65.3) | 38 (46.3) 42 (54.5) |
| Patients with any SAEa | 5 (6.7) 3 (4.0) | 1 (1.2) 2 (2.6) |
| Patients with any treatment-emergent SAE | 6 (8.0) 5 (6.7) | 2 (2.4) 2 (2.6) |
| Deaths | 0 0 | 0 0 |
| Patients with TEAE leading to treatment discontinuationb | 2 (2.7) 0 | 1 (1.2) 0 |
| Nasopharyngitis | 3 (4.0) 4 (5.3) | 0 2 (2.6) |
| Headache | 4 (5.3) 4 (5.3) | 5 (6.1) 4 (5.2) |
| COVID-19 | 4 (5.3) 0 | 1 (1.2) 1 (1.3) |
| Neurodermatitis | 6 (8.0) 1 (1.3) | 3 (3.7) 2 (2.6) |
| Herpes viral infections (HLT)c | 0 0 | 0 4 (5.2) |
| Skin infections (excluding herpetic infections)d | 7 (9.3) 2 (2.7) | 5 (6.1) 4 (5.2) |
| Conjunctivitis (narrow)e | 2 (2.7) 2 (2.7) | 0 3 (3.9) |
| Coronavirus infections (HLT)f | 4 (5.3) 1 (1.3) | 3 (3.7) 1 (1.3) |
| COVID-19 | 4 (5.3) 0 | 1 (1.2) 1 (1.3) |
| Asymptomatic COVID-19 | 0 0 | 2 (2.4) |
| COVID-19 pneumonia | 0 1 (1.3) | 0 0 |
aConsidered unrelated to the study intervention except for two events of sepsis and mesenteritis, experienced by one placebo-treated patient in PRIME. bIn PRIME, one event each of Hodgkin’s disease and neurodermatitis (MedDRA PTs), considered unrelated to the study drug. In PRIME2, one event of urticaria. cHerpes viral infections (HLT) includes MedDRA PTs oral herpes, herpes zoster, ophthalmic herpes zoster and genital herpes simplex. None of these TEAEs was severe, and all patients recovered with corrective treatment while continuing dupilumab. dSkin infection TEAEs (excluding herpetic infections) were identified based on blinded medical review of all reported TEAEs identified as possible skin infections using CMQ30067. This search included MedDRA PTs under HLGT Skin and subcutaneous tissue infections and infestations, all MedDRA PTs under HLT Skin structures and soft tissue infections, all MedDRA PTs of ‘wound infection’ and MedDRA PTs of chalazion, hordeolum and skin papilloma. eConjunctivitis (narrow term) includes MedDRA PTs conjunctivitis, conjunctivitis bacterial, conjunctivitis viral, conjunctivitis adenoviral, conjunctivitis allergic and atopic keratoconjunctivitis. fCoronavirus infections (HLT) include MedDRA PTs COVID-19, asymptomatic COVID-19 and COVID-19 pneumonia. COVID-19, Coronavirus Disease 2019; HLGT, High-Level Group Term; HLT, High level term; MedDRA, Medical Dictionary for Regular Activities; PT, Preferred Term; SAE, serious adverse event; TEAE, treatment-emergent adverse event. Yosipovitch G, et al. Nat Med. 2023;29:1180-1190.
Administration
Indication18
Dupixent is indicated for the treatment of adults with moderate-to-severe PN who are candidates for systemic therapy.
Administration considerations in PN‡
- Rotate injection site with each injection
- Provide proper training to patients and/or caregivers on the preparations and administration of DUPIXENT prior to use, according to the Instructions for Use
- Consider completing all age-appropriate vaccinations as recommended by current immunisation guidelines prior to initiating treatment with DUPIXENT
- If an every-2-week dose is missed, instruct the patients and/or caregivers to administer the injection within 7 days from the missed dose and then resume their original schedule. If the missed dose is not administered within 7 days, instruct the patients and/or caregivers to wait until the next dose on the original schedule
- If an every-4-week dose is missed, instruct the patients and/or caregivers to administer the injection within 7 days from the missed dose and then resume their original schedule. If the missed dose is not administered within 7 days, instruct the patients and/or caregivers to administer the dose, starting a new schedule based on this date
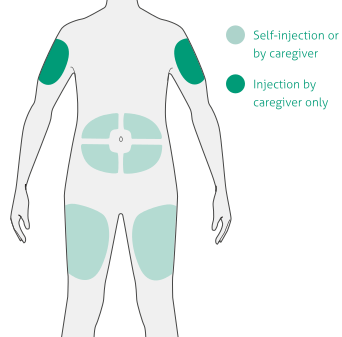
Select the injection site18:
- Stomach (except for 5cm area around navel)
- Thigh
- Outer upper arm (caregiver only)
- Do not inject into skin that is tender, damaged, bruised, or scarred
- Do not inject through clothes
- Wash hands and clean the injection site with an alcohol wipe before injecting—do not touch the injection site again or blow on it
‡Before administering DUPIXENT read complete Instructions for Use.
DUPIXENT offers 2 administration options
DUPIXENT pre-filled syringe18:
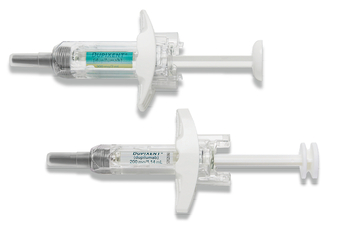
- Manual control of injection speed
- Finger grip for comfort
- Visual confirmation of injection delivery
- Needle shield
- Easy-to-carry format
DUPIXENT pre-filled pen18:
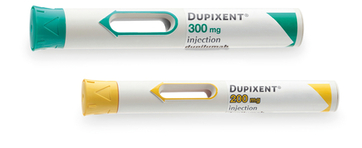
- Single-press auto-injector
- A clear, 2-step process
- Visual and audible feedback
- Hidden needle
- Compact and convenient to carry
- Keep the pre-filled pen(s) or syringe(s) and all medicines out of the reach of children18
- Keep unused pre-filled pens or syringes in the original carton and store in the refrigerator between 2°C and 8°C18
- Do not keep pre-filled pens or syringes at room temperature (<25°C) for more than 14 days18
- Do not shake the pre-filled pen or syringe at any time18
- Do not heat the pre-filled pen or syringe18
- Do not freeze the pre-filled pen or syringe18
- Do not place the pre-filled pen or syringe into direct sunlight18
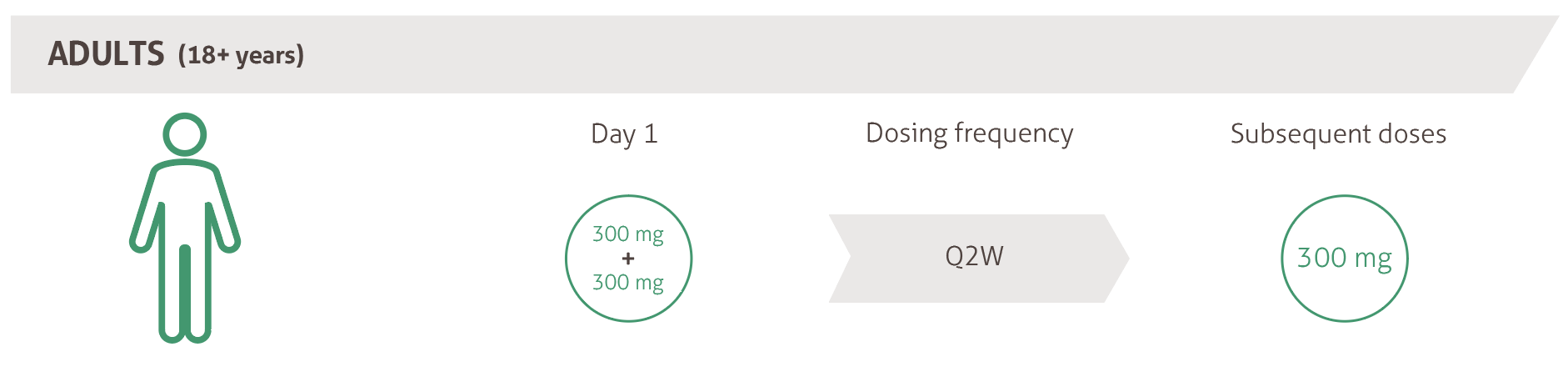
Dosing18
AD, atopic dermatitis; CPD, continuing professional development; DLQI, Dermatology Life Quality Index; IGA PN-S, Investigator’s Global Assessment PN-Stage; IL, interleukin; LS, least squares; PN, prurigo nodularis; QoL, quality of life; SE, standard error; Th, T helper; WI-NRS, Worst Itch Numeric Rating Scale.
Footnotes
*Results from an anonymous survey distributed via PN patient support groups; 171 subjects with PN were included in the final analysis.1
✝Results from a questionnaire study assessing sleep disturbance over multiple time points in 39 subjects with PN.2
‡Results from a questionnaire study to assess treatment patterns, comorbidities, pruritus and QoL in 52 subjects with PN.9
§Results from a prospective, cross-sectional, cohort questionnaire study in 406 subjects with PN from 15 dermatological centres across Europe.8
References
-
Aggarwal P, et al. Clin Exp Dermatol. 2021;46(7):1277-1284.
-
Gwillim EC, et al. Acta Derm Venereol. 2021 ;101(3):adv00424.
-
Whang KA, et al. J Am Acad Dermatol. 2021;S0190-9622(21)01028–8.
-
Jørgensen KM, et al. J Eur Acad Dermatol Venereo. 2017;31(2):e106-e107.
-
Bewley A, et al. Dermatol Ther (Heidelb). 2022;12(9):2039-2048.
-
Pereira MP, et al. J Eur Acad Dermatol Venereol. 2018;32(12):2224-2229.
-
Ständer H, et al. J Am Acad Dermatol. 2020;82(2):460-468.
-
Pereira MP, et al. Acta Derm Venereol. 2021;101(2):adv0403.
-
Todberg T, et al. Acta Derm Venereol. 2020;100(8):adv00119.
-
Satoh T, et al. J Dermatol. 2021;48(9):e414-e431.
-
Belzberg M, et al. J Allergy Clin lmmunol. 2022;149(4):1329–1339.
-
Garcovich S, et al. Vaccines (Basel). 2021;9(3):303.
-
Williams KA, et al. J Am Acad Dermatol. 2020;83(6):1567–1575.
-
Oetjen LK, et al. Cell. 2017;171(1):217–228.
-
Silverberg JI, et al. Dermatol Clin. 2017;35(3):327- 334.
-
Weigelt N, et al. J Cutan Pathol. 2010;37(5):578- 586.
-
Zeidler C, et al. Front Med (Lausanne). 2021;8:649332.
-
DUPIXENT Summary of Product Characteristics. Date last accessed: December 2025.
-
Blauvelt A, et al. Lancet. 2017;389:2287-2303.
-
Guttman-Yassky E, et al. J Allergy Clin lmmunol. 2019;143:155-172.
-
Yosipovitch G, et al. Nat Med. 2023;10.1038/s41591-023-02320-9.
-
Serezani APM, et al. J Allergy Clin lmmunol. 2017;139(1):142-151.
-
Nguyen JK, et aI. Arch Dermatol Res. 2020;312(2):81-92.
-
Yosipovitch G, et al. Nat Med. 2023;10.1038/s41591-023-02320 -9 [Supplementary Material].
-
Clinical Review Report Dupilumab (Dupixent): (Sanofi-Aventis Canada Inc.): Indication: Moderate-to-severe atopic dermatitis (AD) [Internet ]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2018 Jul. Appendix 5, Validity of Outcomes Measures. Available at: https://www.ncbi.nlm.nih.gov/books/NBK539234/. Date last accessed: December 2025.
-
Scottish Medicines Consortium. Dupilumab (DUPIXENT). Available at: https://www.scottishmedicines.org.uk/medicines-advice/dupilumab-dupixent-full-smc2598/. Date last accessed. December 2025.
-
NICE guidance. Dupilumab for treating moderate to severe prurigo nodularis. Available at: https://www.nice.org.uk/guidance/ta955/chapter/1-Recommendations. Date last accessed: December 2025.
-
All Wales Medicines Strategy Groups. Dupilumab (DUPIXENT). Available at: https://awttc.nhs.wales/accessing-medicines/medicine-recommendations/dupilumab-dupixent7/. Date last accessed: December 2025.
-
Northern Ireland Formulary Dupilumab (DUPIXENT). Available at: https://niformulary.hscni.net/managed-entry/managed-entry-decisions/. Date last accessed: December 2025.
MAT-XU-2400765 (v3.0) Date of Preparation December 2025