
Profile

A simple way to help protect adults against flu with one vaccine for adults of all ages1,2
Supemtek TIVr is indicated for active immunisation for the prevention of influenza disease in adults, and should be used in accordance with official recommendations.1 To learn more about the burden of flu click here.
SIMPLE
With one vaccine for adults of all ages2, Supemtek TIVr has the potential to simplify your flu vaccination clinic.
EFFECTIVE
Supemtek TIVr+ has head-to-head efficacy data against another flu vaccine, demonstrating 30% relative risk reduction (1% absolute risk reduction) against flu vs SD-QIVe in adults aged ≥50 years.1,3
FIRST-LINE RECOMMENDED
Joint first-line JCVI recommended across all reimbursable adults cohorts with strengthening evidence of additional benefit.2*
UKHSA data has indicated a 40% and 24% absolute effectiveness against hospitalisation for Supemtek TIVr+ for the 2022/23 and 2023/24 seasons respectively (absolute effectiveness of aIIV was 24% & 17% respectively) for those aged ≥65 years.4**
SIMPLE
With one vaccine for adults of all ages2, Supemtek TIVr has the potential to simplify your flu vaccination clinic.
EFFECTIVE
Supemtek TIVr+ has head-to-head efficacy data against another flu vaccine, demonstrating 30% relative risk reduction (1% absolute risk reduction) against flu vs SD-QIVe in adults aged ≥50 years.1,3
FIRST-LINE RECOMMENDED
Joint first-line JCVI recommended across all reimbursable adults cohorts with strengthening evidence of additional benefit.2*
UKHSA data has indicated a 40% and 24% absolute effectiveness against hospitalisation for Supemtek TIVr+ for the 2022/23 and 2023/24 seasons respectively (absolute effectiveness of aIIV was 24% & 17% respectively) for those aged ≥65 years.4**
+This study used Supemtek (quadrivalent recombinant influenza vaccine), the efficacy of which is relevant to Supemtek TIVr because both vaccines are manufactured using the same process and have overlapping compositions.1
*Recommended for 26/27 flu season
**Confidence intervals overlapped and the difference in point estimates was not statistically significant.
Clinical Evidence
A joint first-line recommended flu vaccine with head-to-head RCT efficacy data against another flu vaccine
Supemtek has shown proven efficacy against another flu vaccine in a head-to-head RCT study, the gold standard of clinical research1,3
Types of study design
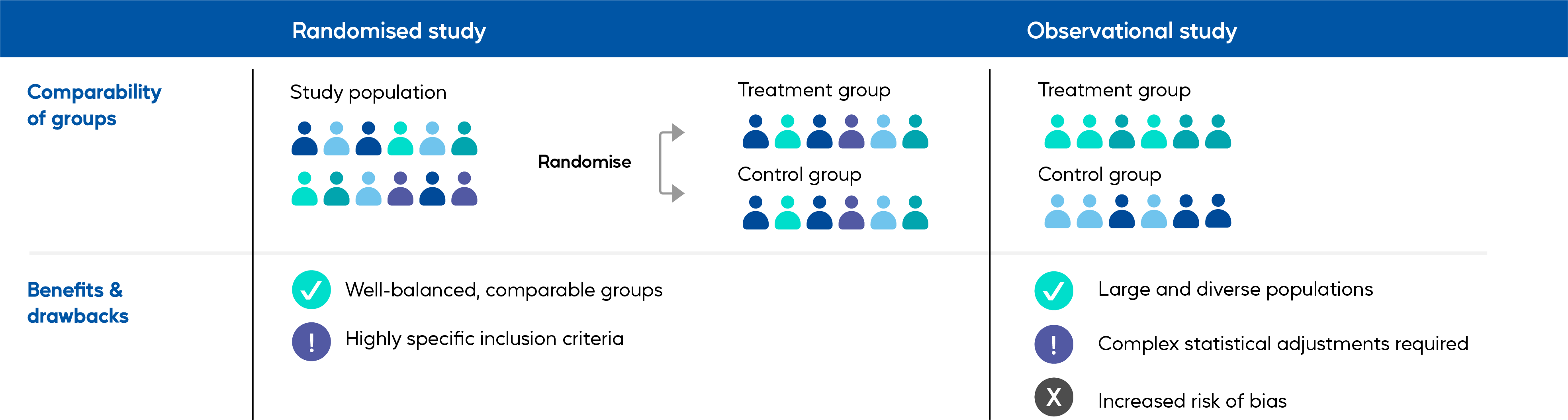
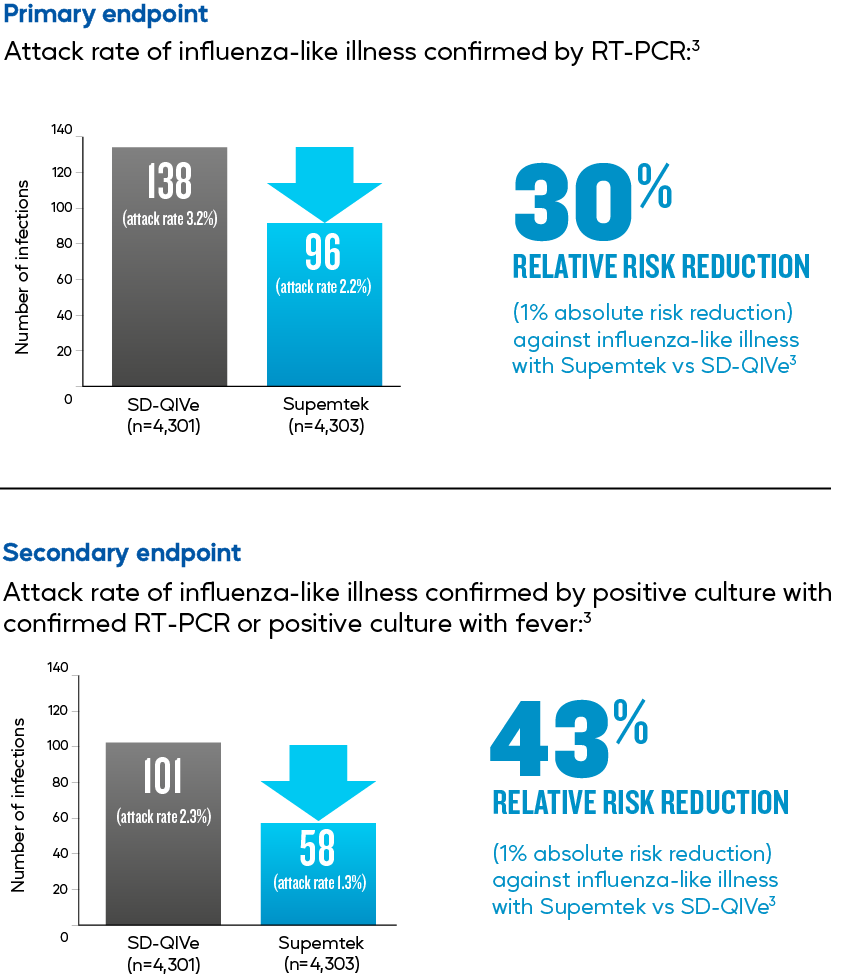
A phase 3-4 randomised controlled trial of 8,604 patients ≥50 years of age was conducted during the A/H3N2-dominant 2014/15 influenza season, and patients were randomised 1:1 to receive a single dose of Supemtek or SD-QIVe. Result based on attack rate of influenza-like illness confirmed by RT-PCR. This study found that Supemtek showed 30% greater relative risk reduction (1% absolute risk reduction) against influenza-like illness versus standard-dose QIVe.
Efficacy of Recombinant Influenza Vaccine in Adults 50 Years of Age or Older
Recent real world data
JCVI would like to see more use of Supemtek TIVr to improve estimates of vaccine effectiveness2
UKHSA vaccine effectivness data, published by Whitaker HJ et al. in Influenza and Other Respiratory Viruses, indicated a 40% and 24% absolute effectiveness against hospitalisation for Supemtek TIVr for the 2022/23 and 2023/24 seasons respectively (absolute effectiveness of aIIV was 24% & 17% respectively) for those aged 65 and over.4*
* Confidence intervals overlapped and the difference in point estimates was not statistically significant.
Usage
With one vaccine for adults for all ages, Supemtek TIVr has the potential to simplify your flu vaccination campaign2
Supemtek TIVr is joint first-line JCVI recommended across all reimbursable adult cohorts.2
Table of JCVI recommended flu vaccines
| Cohort | JCVI advises* | Acceptable alternatives (if advised vaccines are not available) |
|---|---|---|
|
Adults aged |
TIVr (Supemtek TIVr) | |
|
AT-RISK ADULTS |
Supemtek TIVr | TIVe - can also be considered for use in this age group if all other options are unavailable |
Note: there is a limited amount of data on the use of Supemtek TIVr in pregnant women. One animal study performed with trivalent recombinant influenza vaccine did not indicate direct or indirect harmful effects with respect to pregnancy, embryo-foetal development or early post-natal development. An assessment of the risks and benefits should be performed by a healthcare professional before administering Supemtek TIVr to a pregnant woman.2
* Equally suitable options.
Recombinant technology
Supemtek is the first recombinant influenza vaccine in Europe.
Recombinant technology eliminates the possibility of adaptation or mutation by replicating only the haemagglutinin (HA) antigen direct from a genetic sequence. The resulting antigens are an exact genetic match to the target strain HA antigen.5.6
Increased HA content
Supemtek contains 3x more HA antigen (45 mcg versus 15 mcg) per strain, compared with cell and egg-based standard-dose quadrivalent influenza vaccines.1
Adverse Events
Supemtek TIVr offers the reassurance of a known immunogenicity and safety profile1,3
Safety data for Supemtek TIVr are derived from the quadrivalent recombinant influenza vaccine as both vaccines are manufactured using the same process and have overlapping compositions.1,3 The most common reactions that occurred after Supemtek administration were injection-site reactions: tenderness was reported by 48%, and pain by 37%, in study participants 18–49 years of age. In study participants ≥50 years of age, injection site tenderness was reported by 34% and injection site pain by 19%.1,3 Safety and efficacy of Supemtek have not been established in people < 18 years of age.1 The severity of the reactions was mild to moderate. Onset usually occurred within the first 3 days after vaccination. All resolved without sequelae.1 For the full list of adverse events please refer to the Summary of Product Characteristics.
Other adverse events included:1
VERY COMMON≥1/10Headache, fatigue, myalgia, arthralgia, local tenderness, local pain |
COMMON≥1/100 to <1/10Nausea, fever, shivering, chills, firrmness, swelling, redness |
UNCOMMON≥1/1000 to <1/100Cough, oropharyngeal pain, diarrhoea, pruritus, dermatitus, rash, flu-like symptoms, injection site pruritus |
Read more about Influenza:
.

aTIV, (adjuvanted) Trivalent Influenza Vaccine; CI, Confidence Interval; GMT, Geometric Mean Titre; HA, Haemagglutinin; HAI, Haemagglutination Inhibition; HCP, Healthcare Professional; JCVI, Joint Committee on Vaccination and Immunisation; LB, Lower Bound; TIVe, Trivalent Inactivated Influenza; TIVc, Trivalent Influenza Vaccine (cell); TIVr, Trivalent Influenza Vaccine (recombinant); RT-PCR, Reverse-Transcriptase Polymerase Chain-Reaction; RCT, Randomised Controlled Trial; rVE, Relative Vaccine Efficacy; SD-QIVe, Standard Dose Quadrivalent Influenza Vaccine (egg); UB, Upper Bound.
References
- Supemtek TIVr Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/100729/smpc. Accessed December 2025.
- JCVI statement on influenza vaccines for 2026 to 2027. Available at: https://www.gov.uk/government/publications/flu-vaccines-2026-to-2027-jcvi-advice-16-july-2025/jcvi-statement-on-influenza-vaccines-for-2026-to-2027#introduction. Last accessed: December 2025
- Dunkle LM, et al. N Eng J Med 2017;376;2427-2436
- Whitaker HJ et al, Influenza Other Respir Viruses, 2025, 19(12):1-13
- Centers for Disease Control and Prevention. How influenza (flu) vaccines are made. https://www.cdc.gov/flu/vaccine-process/index.html. Accessed December 2025
- Dunkle LM, et al. J Infect Dis. 2017;216:1219-26
MAT-XU-2502441 (v3.0) Date of preparation: December 2025
