- Article
- Source: Campus Sanofi
- 22 Oct 2023
Toujeo (insulin glargine 300 Units/mL) Versus insulin degludec 100 units/mL in T1DM
.jpg)
Prescribing Information can be found through the links in the Product Cards at the bottom of the page
Toujeo® versus insulin degludec 100 units/mL in T1DM
InRange - randomised, active-controlled, open-label study4,5
Primary endpoint was met: non-inferiority to insullin degludec 100 units/mL for time in range (TIR) ≥70 to ≤180 mg/dL (≥3.9 to ≤10 mmol/L) at Week 125
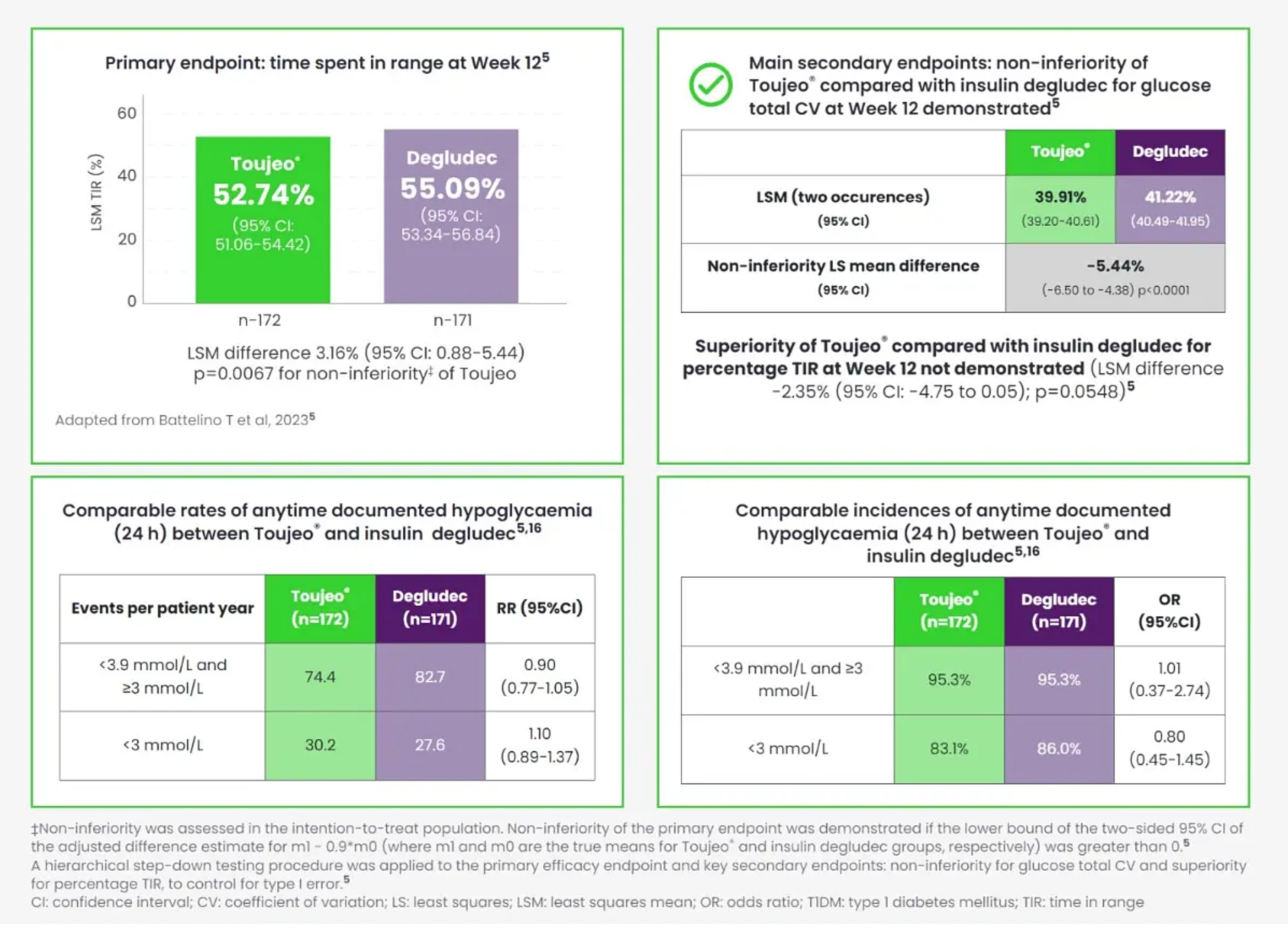updated.jpg)
Professor Richard Bergenstal presents the results of the InRange study. This study is the first randomised controlled trial to compare second generation basal insulin analogues Toujeo® and insulin degludec 100 U/mL. It compares adults with T1DM using Time-In-Range (TIR) as the primary endpoint. Watch and learn about the study design and key findings
.png)
View the guide to find out more about the impact of time in range on your patients
.png)
View the guide to find out more about the impact of time in range on your patients
Hypoglycaemia is the most frequent adverse reaction observed in clinical trials conducted with Toujeo®.7 Other common adverse effects are lipohypertrophy and injection site reactions.7
For further information on the safety profile of Toujeo® please consult the Summary of Product Characteristics >
ARR: Absolute Risk Reduction
HR: Hazard Ratio
OR: Odds Ratio
RR: Relative Risk
T1D – Type-1 Diabetes
T2D – Type -2 Diabetes
Diabetes Products
References
- Danne T, Matsuhisa M et al. Lower risk of severe hypoglycaemia with insulin glargine 300 U/mL versus glargine 100 U/mL in participants with type 1 diabetes: a meta-analysis of 6-month phase 3 clinical trials. Diabetes Obes Metab 2020;22(10):1880-1885
- Ritzel R, Roussel R et al. Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab 2015;17(9):859-867
- Ritzel R, Roussel R et al. Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes. Diabetes Obes Metab 2018;20(3):541-548
- Battelino T, Bosnyak Z et al. InRange: comparison of the second-generation basal insulin analogues glargine 300 u/ml and degludec 100 u/ml in persons with type 1 diabetes using continuous glucose monitoring-study design. Diabetes Ther 2020;11(4):1017-1027
- Battelino T, Danne T et al. Continuous glucose monitoring-based time-in-range using insulin glargine 300 units/ml versus insulin degludec 100 units/ml in type 1 diabetes: the head-to-head randomised controlled InRange trial. Diabetes Obes Metab 2023;25(2):545-555
- Rosenstock J, Cheng A et al. More similarities than differences testing insulin glargine 300 units/ml versus insulin degludec 100 units/ml in insulin-naive type 2 diabetes: the randomised head-to-head BRIGHT trial. Diabetes Care 2018;41(10):2147-2154
- Toujeo Summary of Product Characteristics
- Danne T, Tamborlane W V et al. Efficacy and safety of insulin glargine 300 units/ml (gla-300) versus insulin glargine 100 units/ml (gla-100) in children and adolescents (6-17 years) with type 1 diabetes: results of the EDITION JUNIOR randomised controlled trial. Diabetes Care 2020;43(7):1512-1519
- Home P D, Bergenstal R M et al. New insulin glargine 300 units/ml versus glargine 100 units/ml in people with type 1 diabetes: a randomised, phase 3a, open-label clinical trial (EDITION 4). Diabetes Care 2015;38(12):2217-2225
- Matsuhisa M, Koyama M et al. New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese adults with type 1 diabetes using basal and mealtime insulin: glucose control and hypoglycaemia in a randomised controlled trial (EDITION JP 1). Diabetes Obes Metab 2016;18(4):375-383
- Danne T, Matsuhisa M et al. Lower risk of severe hypoglycaemia with insulin glargine 300 U/mL versus glargine 100 U/mL in participants with type 1 diabetes: a meta-analysis of 6-month phase 3 clinical trials. Diabetes Obes Metab 2020;22(10):1880-1885 (suppl)
- Bolli G B, Riddle M C et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naive people with type 2 diabetes on oral glucose-lowering drugs: a randomised controlled trial (EDITION 3). Diabetes Obes Metab 2015;17(4):386-394
- Riddle M C, Bolli G B et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomised controlled trial (EDITION 1). Diabetes Care 2014;37(10):2755-2762
- Yki-Jarvinen H, Bergenstal R et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomised controlled trial (EDITION 2). Diabetes Care 2014;37(12):3235-3243
- Ritzel R, Roussel R et al. Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes. Diabetes Obes Metab 2018;20(3):541-548 (suppl)
- Battelino T, Danne T et al. Time in range using insulin glargine 300 U/mL versus insulin degludec 100 U/mL in type 1 diabetes: head-to-head randomised controlled InRange trial. Presented at Advanced Technologies and Treatments for Diabetes conference, Barcelona, Spain, 27-30 April 2022; abstract LB009/ #1015
- Rosenstock J, Cheng A et al. More similarities than differences testing insulin glargine 300 units/ml versus insulin degludec 100 units/ml in insulin-naive type 2 diabetes: the randomised head-to-head BRIGHT trial. Diabetes Care 2018;41(10):2147-2154 (suppl)
- Cheng A, Harris S et al. Similar glycaemic control and less hypoglycaemia during active titration after insulin initiation with glargine 300 units/mL and degludec 100 units/mL: a subanalysis of the BRIGHT study. Diabetes Obes Metab 2020;22(3):346-354
- Sanofi. Efficacy and safety of Toujeo® versus Tresiba® in insulin-naïve patients with type 2 diabetes mellitus inadequately controlled with oral antihyperglycemic drug(s) ± glp-1 receptor agonist (BRIGHT). Available at: clinicaltrials.gov. Accessed October 2024
- Bolli G B, Cheng A. Lower hypoglycaemia rates with insulin glargine 300 U/ml vs insulin degludec 100 U/ml in insulin-naive adults with T2DM: the BRIGHT randomised trial. Presented at EASD, Berlin, Germany, 1-5 October 2018; poster 896
MAT-XU-2302855 (v5.0) Date of Preparation: October 2024

