- Article
- Source: Campus Sanofi
- 22 Oct 2023
Toujeo (insulin glargine 300 Units/mL) Versus insulin glargine 100 units/mL in T1DM
.jpg)
Prescribing Information can be found through the links in the Product Cards at the bottom of the page
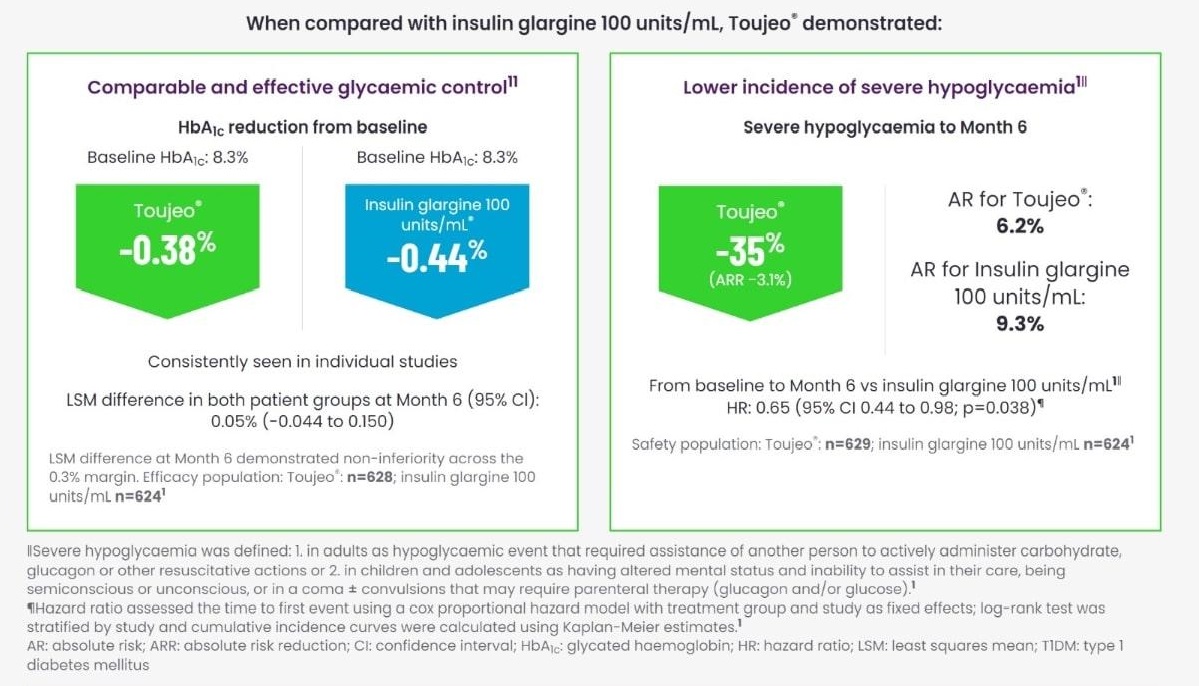
Toujeo® versus insulin glargine 100 units/mL in T1DM
EDITION 4, EDITION JUNIOR and EDITION JP1 - meta-analysis1,8-10
All individual trials met their primary endpoints: change in HbA1c from baseline to Week 268-10
%20updated.2178623535607379947.jpg)
.png)
Clinical summary to read about the benefits of Toujeo® in children and adolescents from the age of 6 years in this randomised trial
%20(6).png)
This slide deck can be used to support Health Care Professional training on Toujeo. It provides an overview of clinical studies T1 Edition Junior and Meta-Analysis
.png)
Clinical summary to read about the benefits of Toujeo® in children and adolescents from the age of 6 years in this randomised trial
%20(6).png)
This slide deck can be used to support Health Care Professional training on Toujeo. It provides an overview of clinical studies T1 Edition Junior and Meta-Analysis
Hypoglycaemia is the most frequent adverse reaction observed in clinical trials conducted with Toujeo®.7 Other common adverse effects are lipohypertrophy and injection site reactions.7
For further information on the safety profile of Toujeo® please consult the Summary of Product Characteristics >
ARR: Absolute Risk Reduction
HR: Hazard Ratio
OR: Odds Ratio
RR: Relative Risk
T1D – Type-1 Diabetes
T2D – Type -2 Diabetes
Diabetes Products
References
- Danne T, Matsuhisa M et al. Lower risk of severe hypoglycaemia with insulin glargine 300 U/mL versus glargine 100 U/mL in participants with type 1 diabetes: a meta-analysis of 6-month phase 3 clinical trials. Diabetes Obes Metab 2020;22(10):1880-1885
- Ritzel R, Roussel R et al. Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab 2015;17(9):859-867
- Ritzel R, Roussel R et al. Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes. Diabetes Obes Metab 2018;20(3):541-548
- Battelino T, Bosnyak Z et al. InRange: comparison of the second-generation basal insulin analogues glargine 300 u/ml and degludec 100 u/ml in persons with type 1 diabetes using continuous glucose monitoring-study design. Diabetes Ther 2020;11(4):1017-1027
- Battelino T, Danne T et al. Continuous glucose monitoring-based time-in-range using insulin glargine 300 units/ml versus insulin degludec 100 units/ml in type 1 diabetes: the head-to-head randomised controlled InRange trial. Diabetes Obes Metab 2023;25(2):545-555
- Rosenstock J, Cheng A et al. More similarities than differences testing insulin glargine 300 units/ml versus insulin degludec 100 units/ml in insulin-naive type 2 diabetes: the randomised head-to-head BRIGHT trial. Diabetes Care 2018;41(10):2147-2154
- Toujeo Summary of Product Characteristics
- Danne T, Tamborlane W V et al. Efficacy and safety of insulin glargine 300 units/ml (gla-300) versus insulin glargine 100 units/ml (gla-100) in children and adolescents (6-17 years) with type 1 diabetes: results of the EDITION JUNIOR randomised controlled trial. Diabetes Care 2020;43(7):1512-1519
- Home P D, Bergenstal R M et al. New insulin glargine 300 units/ml versus glargine 100 units/ml in people with type 1 diabetes: a randomised, phase 3a, open-label clinical trial (EDITION 4). Diabetes Care 2015;38(12):2217-2225
- Matsuhisa M, Koyama M et al. New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese adults with type 1 diabetes using basal and mealtime insulin: glucose control and hypoglycaemia in a randomised controlled trial (EDITION JP 1). Diabetes Obes Metab 2016;18(4):375-383
- Danne T, Matsuhisa M et al. Lower risk of severe hypoglycaemia with insulin glargine 300 U/mL versus glargine 100 U/mL in participants with type 1 diabetes: a meta-analysis of 6-month phase 3 clinical trials. Diabetes Obes Metab 2020;22(10):1880-1885 (suppl)
- Bolli G B, Riddle M C et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naive people with type 2 diabetes on oral glucose-lowering drugs: a randomised controlled trial (EDITION 3). Diabetes Obes Metab 2015;17(4):386-394
- Riddle M C, Bolli G B et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomised controlled trial (EDITION 1). Diabetes Care 2014;37(10):2755-2762
- Yki-Jarvinen H, Bergenstal R et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomised controlled trial (EDITION 2). Diabetes Care 2014;37(12):3235-3243
- Ritzel R, Roussel R et al. Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes. Diabetes Obes Metab 2018;20(3):541-548 (suppl)
- Battelino T, Danne T et al. Time in range using insulin glargine 300 U/mL versus insulin degludec 100 U/mL in type 1 diabetes: head-to-head randomised controlled InRange trial. Presented at Advanced Technologies and Treatments for Diabetes conference, Barcelona, Spain, 27-30 April 2022; abstract LB009/ #1015
- Rosenstock J, Cheng A et al. More similarities than differences testing insulin glargine 300 units/ml versus insulin degludec 100 units/ml in insulin-naive type 2 diabetes: the randomised head-to-head BRIGHT trial. Diabetes Care 2018;41(10):2147-2154 (suppl)
- Cheng A, Harris S et al. Similar glycaemic control and less hypoglycaemia during active titration after insulin initiation with glargine 300 units/mL and degludec 100 units/mL: a subanalysis of the BRIGHT study. Diabetes Obes Metab 2020;22(3):346-354
- Sanofi. Efficacy and safety of Toujeo® versus Tresiba® in insulin-naïve patients with type 2 diabetes mellitus inadequately controlled with oral antihyperglycemic drug(s) ± glp-1 receptor agonist (BRIGHT). Available at: clinicaltrials.gov. Accessed October 2024
- Bolli G B, Cheng A. Lower hypoglycaemia rates with insulin glargine 300 U/ml vs insulin degludec 100 U/ml in insulin-naive adults with T2DM: the BRIGHT randomised trial. Presented at EASD, Berlin, Germany, 1-5 October 2018; poster 896
MAT-XU-2302853 (v6.0) Date of Preparation: October 2024

