- Article
- Source: Campus Sanofi
- 24 May 2024
Toujeo (insulin glargine 300 Units/mL) in addition to GLP1-RA

Prescribing Information can be found through the links in the Product Cards at the bottom of the page
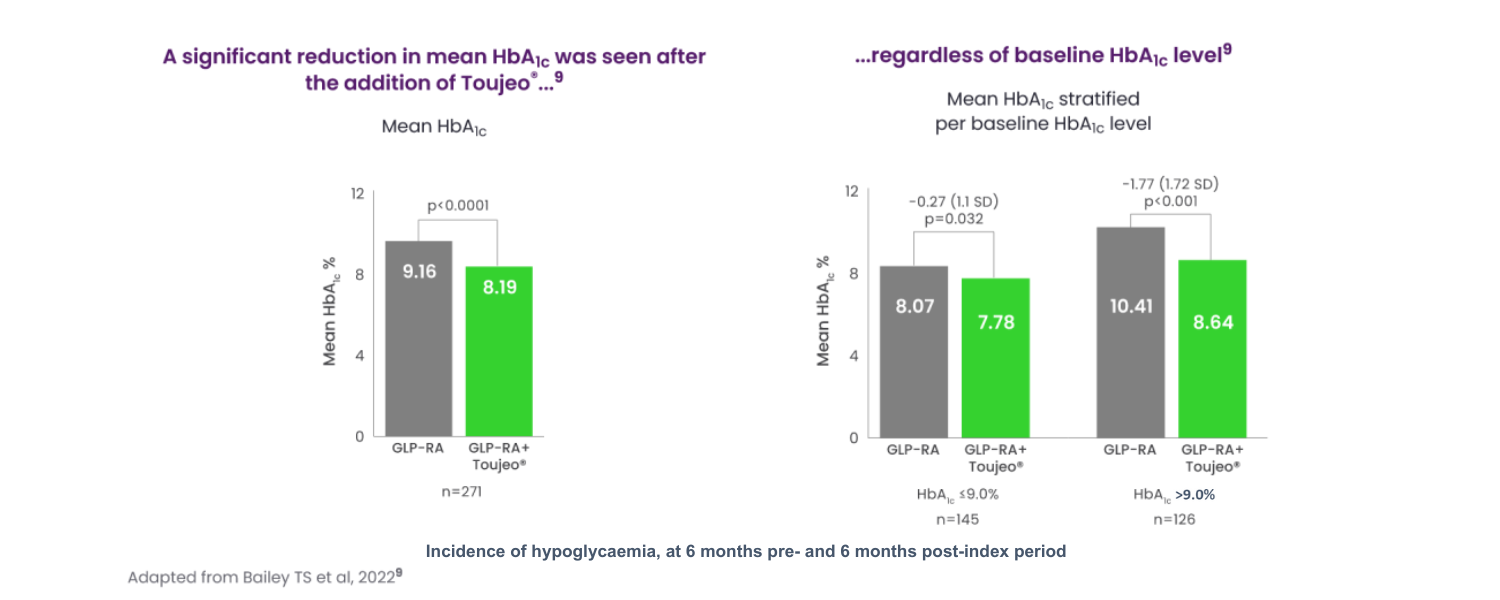
Addition of Toujeo® to GLP-1 RA was associated with significantly improved glycaemic control.9
DELIVER-G real-world retrospective analysis.
A real-world retrospective analysis in insulin-naïve adults with T2D inadequately controlled on GLP‑1 RA with or without oral antidiabetic drugs (N=271).9
DELIVER-G was a retrospective analysis of 271 people with Type 2 diabetes receiving GLP‑1 RA therapy who were insulin‑naive prior to subsequent treatment intensification with Toujeo® (insulin glargine 300 U/mL). DELIVER‑G analysed data from a large US electronic medical record data source (IBM® Explorys).9
Hypoglycaemia is the most frequent adverse reaction observed in clinical trials conducted with Toujeo®.7 Other common adverse effects are lipohypertrophy and injection site reactions.7
For further information on the safety profile of Toujeo® please consult the Summary of Product Characteristics >
ARR: Absolute Risk Reduction
HR: Hazard Ratio
OR: Odds Ratio
RR: Relative Risk
T1D – Type-1 Diabetes
T2D – Type -2 Diabetes
9. Bailey T S, Gill J et al. Real-world outcomes of addition of insulin glargine 300 U/mL (Gla-300) to glucagon-like peptide-1 receptor agonist (GLP-1 RA) therapy in people with type 2 diabetes: The DELIVER-G study. Diabetes Obes Metab 2022;24(8):1617-1622
Diabetes Products
MAT-XU-2402513 (v1.0) Date of Preparation: October 2024

