Qfitlia versus on-demand therapies1*
reduction in ABR with Qfitlia versus factor replacement on-demand in patients without inhibitors.1
• Mean ABR: 9.0 vs 31.4, respectively, P<.0001†
reduction in ABR with Qfitlia versus BPA on-demand in patients with inhibitors.1
• Mean ABR: 5.1 vs 19.1, respectively, P=.0006†
* The integrated efficacy analyses were conducted according to the ITT principle, preserving the randomization from the parent studies. The efficacy of Qfitlia was evaluated in 177 patients, comparing Qfitlia prophylaxis under the AT-DR in ATLAS-OLE and BPA or factor replacement on-demand in the parent studies.1,2
† Based on treated bleeds.2
Qfitlia ABR following prior administration of prophylaxis treatment in people with hemophilia A and B1,2
In patients without inhibitors on prior prophylaxis:
• Estimated mean ABR with Qfitlia prophylaxis versus prior factor prophylaxis: 6.93 vs 5.96, respectively
— The estimated mean ABR observed with Qfitlia was comparable to prior factor prophylaxis*
In patients with inhibitors on prior prophylaxis:
• Estimated mean ABR with Qfitlia prophylaxis versus prior BPA prophylaxis: 3.41 vs 11.29, respectively
— The estimated mean ABR observed with Qfitlia was reduced versus prior BPA prophylaxis*
This data is exploratory in nature and not statistically proven. Results are descriptive, and definitive conclusions cannot be made.
* Data from 69 patients treated with Qfitlia prophylaxis under the AT-DR in ATLAS-OLE compared to their prior treatment with BPA or factor prophylaxis in the ATLAS-PPX study.2
Bleed rates of Qfitlia across subgroups in the ATLAS-OLE study1
ABR and AsBR across patient types1,3*
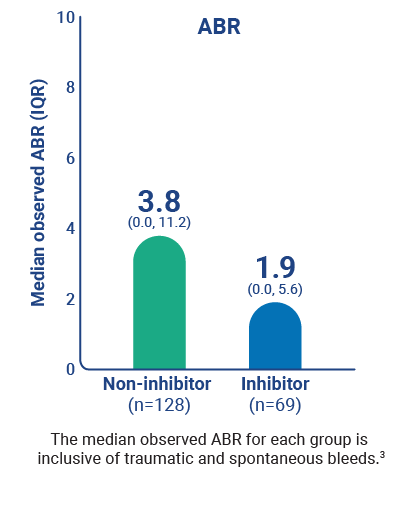
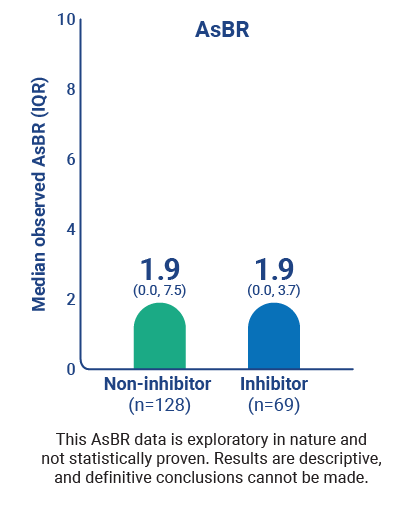
Percentage of patients on Qfitlia who experienced 1 or fewer bleeds3
of patients experienced 0 bleeds
of patients experienced 0 or 1 bleed
This data is exploratory in nature and not statistically proven. Results are descriptive, and definitive conclusions cannot be made.
* Based on treated bleeds.3
INDICATION
ABR=annualized bleed rate; AsBR=annualized spontaneous bleed rate; AT-DR=antithrombin-based dosing regimen; BPA=bypassing agent; IQR=interquartile range; ITT=intent-to-treat; OLE=open-label extension.
References: 1. Qfitlia Prescribing Information. Genzyme Corporation. Cambridge, MA. 2. Young G, et al; for the ATLAS-OLE Trial Group. Safety and efficacy of the fitusiran revised antithrombin-based dose regimen (AT-DR) in people with haemophilia (PwH) A or B, with or without inhibitors (ATLAS-OLE). Presented at: European Association for Haemophilia and Allied Disorders Annual Congress; February 6-9, 2024; Frankfurt, Germany. 3. Data on file CSR. SAR439774-LTE15174 - fitusiran. November 2023.