Sam, Age 13

Sam is crazy about soccer and aspires to play for his school team, but his hemophilia gets in the way. Sometimes, he gets overwhelmed by his treatment and forgets to infuse. His parents would love to see him play in a match, but they worry about him having a bleed.



Why could Qfitlia be a good fit for patients like Sam?
Qfitlia offered low ABR in people with hemophilia, including those with inhibitors, in the ATLAS-OLE study1
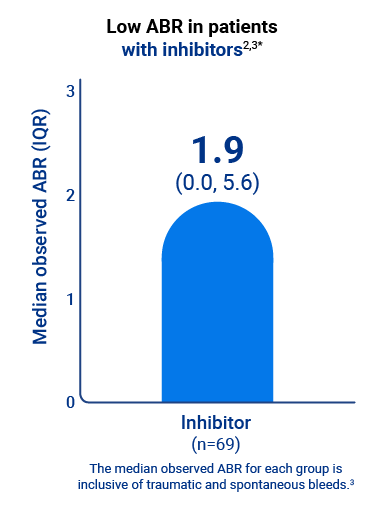
* Based on treated bleeds.3
Robert, Age 21

Robert finds it hard to balance the burden of hemophilia and its treatment with his everyday life. He wants to get on with his life, talk less about his hemophilia, and think less about his treatment. With college finishing soon, and Robert starting his first job, he needs protection that gives him the freedom to live on his terms.


Why could Qfitlia be a good fit for patients like Robert?
Qfitlia offers proven bleed protection with the fewest doses via convenient subcutaneous administration2,4-16
As few as 6 injections a year,* with2:
• Convenient subcutaneous administration via a prefilled pen2†
• Storage at room temperature‡ (for a single period of up to 3 months within the expiration date)2
* In the clinical study of 227 patients, ~67% of people with hemophilia took Qfitlia every other month, and ~19% took it monthly.2
† Vial and syringe administration for the 10 mg and 20 mg dose.2
‡ 15 °C to 30 °C (59 °F to 86 °F).2
Michael, Age 48

Michael loves to travel, both for work and with his family. But when he does, it comes with all sorts of considerations—as well as a lot of stress and anxiety. His current dosing schedule is not a great fit; he needs a treatment that can keep up with him, so he can excel at work and embrace time with his kids.


Why could Qfitlia be a good fit for patients like Michael?
Qfitlia offers proven bleed protection with as few as 6 injections a year—so Michael can spend less time treating prophylactically and more time traveling with his family.2
Qfitlia offers proven bleed protection with storage requirements that are compatible with life on the go2
Seamless storage and logistics2
Storage at room temperature* (for a single period of up to 3 months within the expiration date)2
Convenient subcutaneous administration via a prefilled pen2†
At-home administration2
* 15 °C to 30 °C (59 °F to 86 °F).2
† Vial and syringe administration for the 10 mg and 20 mg dose.2
INDICATION
ABR=annualized bleed rate; BPA=bypassing agent; IQR=interquartile range.
References: 1. Young G, et al; for the ATLAS-OLE Trial Group. Safety and efficacy of the fitusiran revised antithrombin-based dose regimen (AT-DR) in people with haemophilia (PwH) A or B, with or without inhibitors (ATLAS-OLE). Presented at: European Association for Haemophilia and Allied Disorders Annual Congress; February 6-9, 2024; Frankfurt, Germany. 2. Qfitlia Prescribing Information. Genzyme Corporation. Cambridge, MA. 3. Data on file CSR. SAR439774-LTE15174 - fitusiran. November 2023. 4. Advate Prescribing Information. Takeda Pharmaceuticals U.S.A., Inc. Lexington, MA. 5. Adynovate Prescribing Information. Takeda Pharmaceutical U.S.A., Inc. Lexington, MA. 6. Afstyla Prescribing Information. CSL Behring LLC. Kankakee, IL. 7. ALTUVIIIO Prescribing Information. Bioverativ Therapeutics Inc. Waltham, MA. 8. Esperoct Prescribing Information. Novo Nordisk Inc. Plainsboro, NJ. 9. Helixate Prescribing Information. CSL Behring LLC. Kankakee, IL. 10. Hemlibra Prescribing Information. Genentech, Inc. San Francisco, CA. 11. Jivi Prescribing Information. Bayer HealthCare LLC. Whippany, NJ. 12. Kogenate Prescribing Information. Bayer HealthCare LLC. Whippany, NJ. 13. Kovaltry Prescribing Information. Bayer HealthCare LLC. Whippany, NJ. 14. Novoeight Prescribing Information. Novo Nordisk Inc. Plainsboro, NJ. 15. Nuwiq Prescribing Information. Octapharma USA, Inc. Paramus, NJ. 16. Xyntha Prescribing Information. Wyeth Pharmaceuticals LLC. Philadelphia, PA.