The Qfitlia clinical program was studied for over 7 years in people with hemophilia A or B, with or without inhibitors1,2
The ATLAS clinical trial program included two parent studies (ATLAS-INH and ATLAS-A/B), a supportive study (ATLAS-PPX), and an open-label extension study (ATLAS-OLE)3
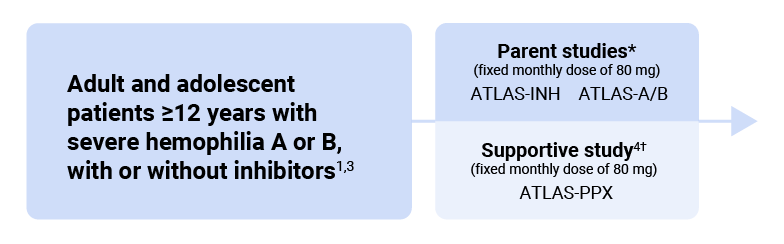
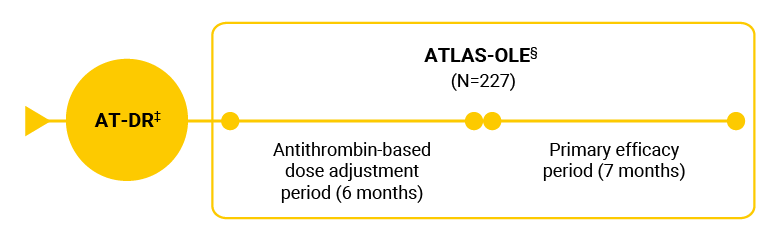
The efficacy of Qfitlia AT-DR treatment was assessed by comparing the Qfitlia AT-DR treatment data from the long-term extension study, ATLAS-OLE, to the control data from studies ATLAS-INH and ATLAS-A/B.1,5
Excluded from the trial were patients with known coexisting coagulation disorders other than hemophilia A or B, an increased risk of thrombosis as assessed by a history of arterial or venous thromboembolism, significant valvular disease or atrial fibrillation, coexisting thrombophilic disorders (eg, Factor V Leiden mutation), antithrombin activity <60% at screening, platelet count ≤100,000/μL, eGFR ≤45 mL/min (using the MDRD), or clinically significant liver disease.3
* ATLAS-INH was a randomized, multicenter, open-label clinical study in 57 adult and pediatric males (aged ≥12 years) with hemophilia A or B with inhibitory antibodies to FVIII or FIX who had previously received on-demand (episodic) treatment with BPAs for bleeding. ATLAS-A/B was a randomized, multicenter, open-label clinical study in 120 adult and pediatric males (aged ≥12 years) with hemophilia A or B without inhibitory antibodies to FVIII or FIX who had previously received on-demand (episodic) treatment with factor replacement for bleeding. Eligible patients in both trials were randomized in a 2:1 ratio to receive Qfitlia prophylaxis (80 mg SC once monthly) or the comparator arm (BPA on-demand or factor replacement on-demand, respectively) to treat breakthrough bleeding episodes for 9 months.3
† ATLAS-PPX was an exploratory, Phase 3, open-label study evaluating the efficacy and safety of Qfitlia prophylaxis in 80 males aged ≥12 years with hemophilia A or B, with or without inhibitors, who had received prior BPA or factor replacement prophylaxis. Participants continued their prior BPA or factor replacement prophylaxis for 6 months before switching to once-monthly 80 mg Qfitlia prophylaxis for 7 months (onset and efficacy periods).4
‡ To mitigate the risk of thrombotic events, an AT-DR with the target antithrombin activity range of 15% to 35% was implemented in ATLAS-OLE when studies ATLAS-INH and ATLAS-A/B were nearly completed.3
§ ATLAS-OLE, a multicenter, multinational, open-label extension study, assessed the safety and efficacy of Qfitlia using dose modifications based on antithrombin activity levels.1,3
The ATLAS-OLE trial included a diverse patient population who were treated with the AT-DR3
Hemophilia A
174 patients1
59 with inhibitors
115 without inhibitors
Hemophilia B
53 patients1
19 with inhibitors
34 without inhibitors
This study included patients between the ages of 12 and 72 years.1,3
INDICATION
AT-DR=antithrombin-based dosing regimen; BPA=bypassing agent; eGFR=estimated glomerular filtration rate; FIX=Factor IX; FVIII=Factor VIII; MDRD=modification of diet in renal disease; SC=subcutaneous.
References: 1. Data on file CSR. SAR439774-LTE15174 - fitusiran. November 2023. 2. Young G, et al. Res Pract Thromb Haemost. 2023;7(4):e100179. 3. Qfitlia Prescribing Information. Genzyme Corporation. Cambridge, MA. 4. Kenet G, et al. Blood. 2024;143(22):2256-2269. 5. Young G, et al; for the ATLAS-OLE Trial Group. Safety and efficacy of the fitusiran revised antithrombin-based dose regimen (AT-DR) in people with haemophilia (PwH) A or B, with or without inhibitors (ATLAS-OLE). Presented at: European Association for Haemophilia and Allied Disorders Annual Congress; February 6-9, 2024; Frankfurt, Germany.
