- Article
- Source: Campus Sanofi
- 16 Jul 2025
Tolebrutinib Direct Effects on Myeloid Cells in PwMS
Understanding Tolebrutinib Fast Fact Series
| Cell-based IC50 1 | ||
| BTK | Microglia-HMC cells | 0.7 nM |
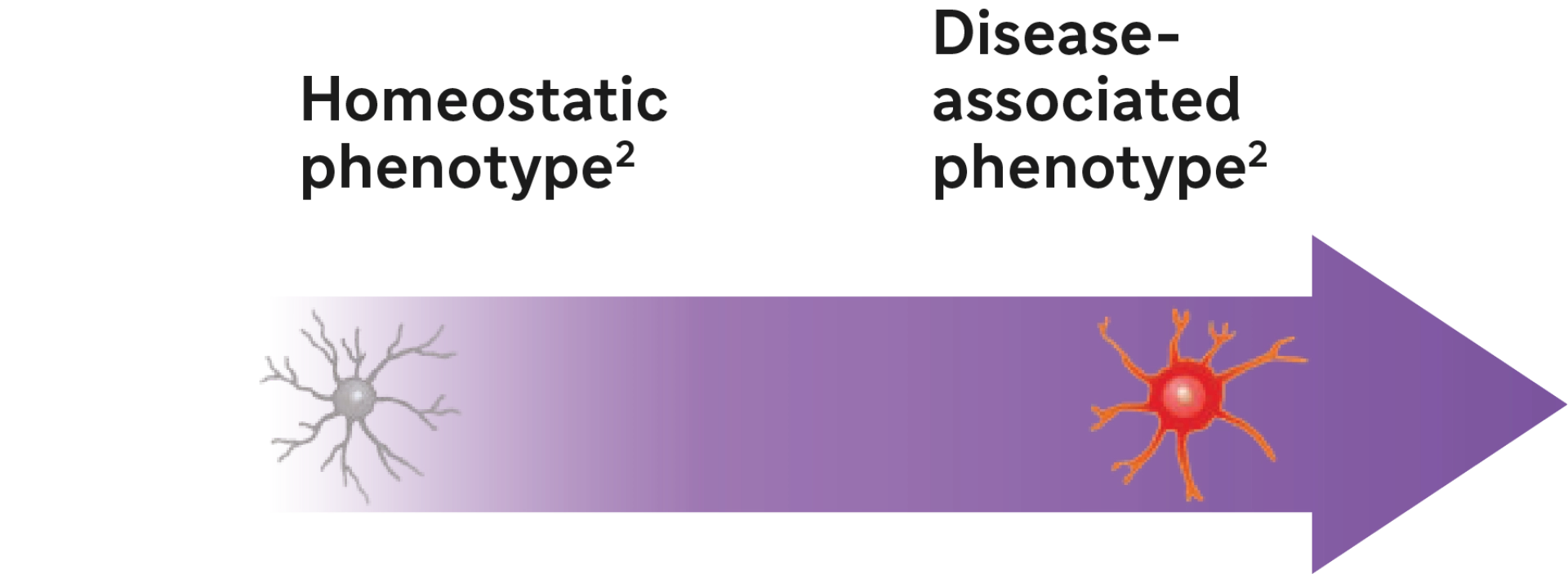
- Tolebrutinib shifted microglia from a disease-associated to a homeostatic phenotype in a mouse model3
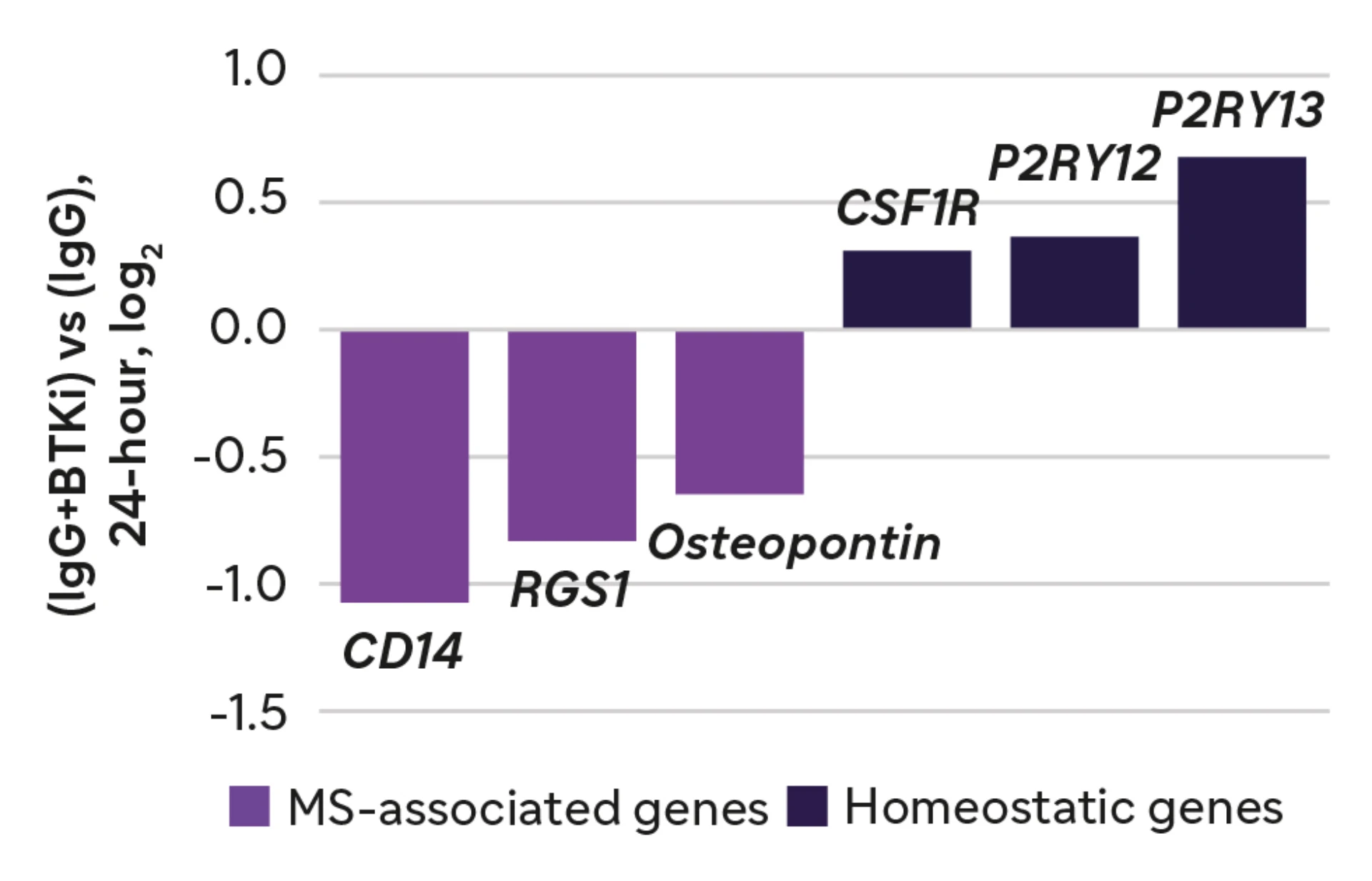
Effect on microglial gene expression in vitro3
|
BRaKe-MS Phase 2a study design4
|
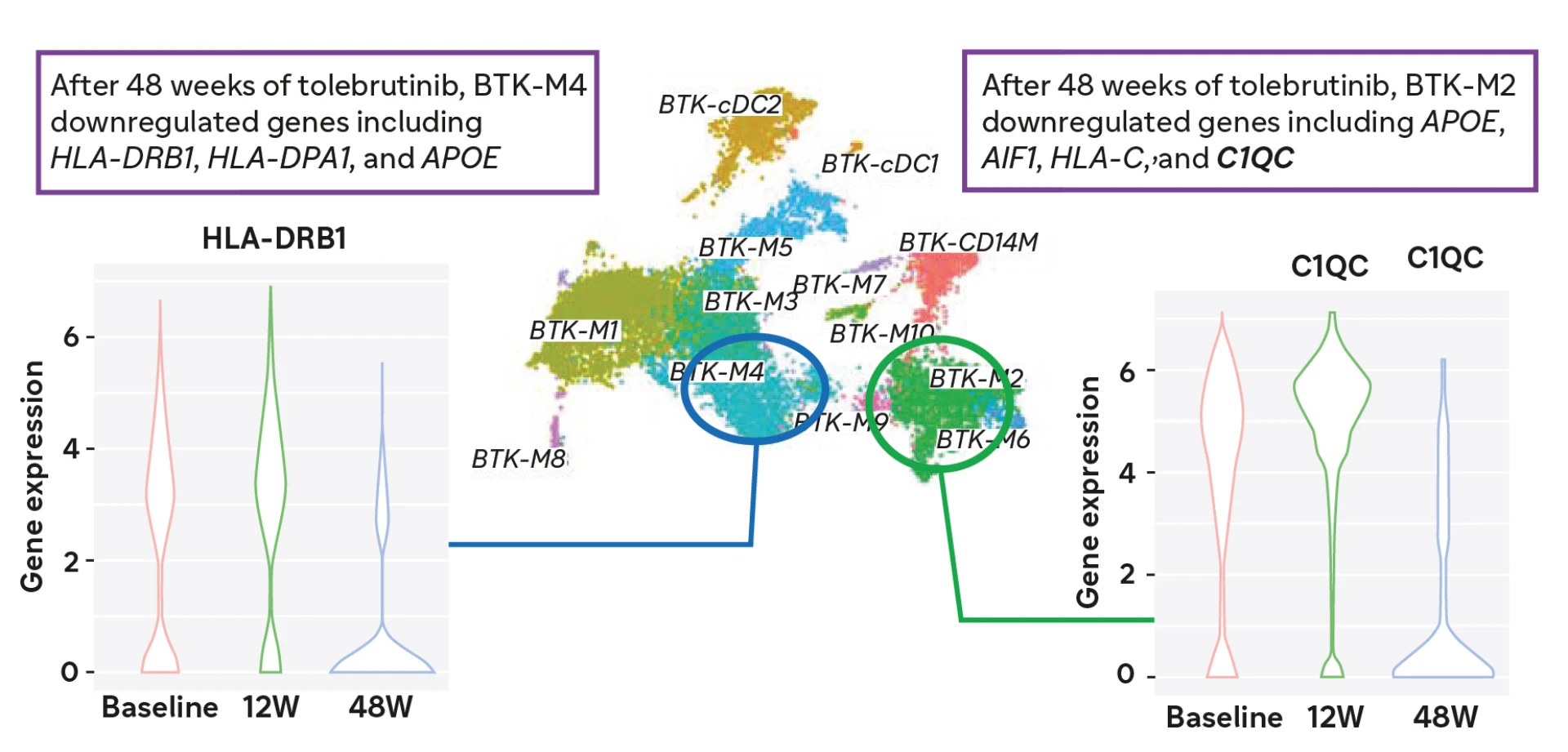
At 48 weeks after crossing over to tolebrutinib, gene expression is altered in myeloid cells4
Tolebrutinib is an investigational agent and has not been approved for use by any regulatory authority.
Tolebrutinib is an investigational agent and has not been approved for use by any regulatory authority.
AIF1=allograft inflammatory factor 1; APOE=apolipoprotein E; BTK=Bruton’s tyrosine kinase; BTKi=BTK inhibitor; C1QC=complement C1Q C chain; CSF=cerebrospinal fluid; CSF1R=colony stimulating factor 1 receptor; HMC=human microglia clone; IC50=50% maximum drug inhibition; HLA=human leukocyte antigen; Ig=immunoglobulin; OPN=osteopontin; P2RY12=purinergic receptor P2Y12; P2RY13=purinergic receptor P2Y13; PwMS=people with MS; RGS1=regulator of G protein signalling 1; W=weeks.
-
Gruber R, et al. AAN 2021, Presentation S25.003.
-
Woodburn SC, et al. J Neuroinflammation. 2021;18:258.
-
Gruber R, et al. ECTRIMS 2021, Poster 391.
-
Raza SA, et al. ECTRIMS 2023, Presentation 1589/O031.
MAT-BH-2500361-V1-June 2025