Dosing
DUPIXENT offers the choice of at‑home or in‑clinic administration
Recommended dosing for adult patients with inadequately controlled severe CRSwNP1

Designed with patients in mind. DUPIXENT has 2 self-administration options1,2
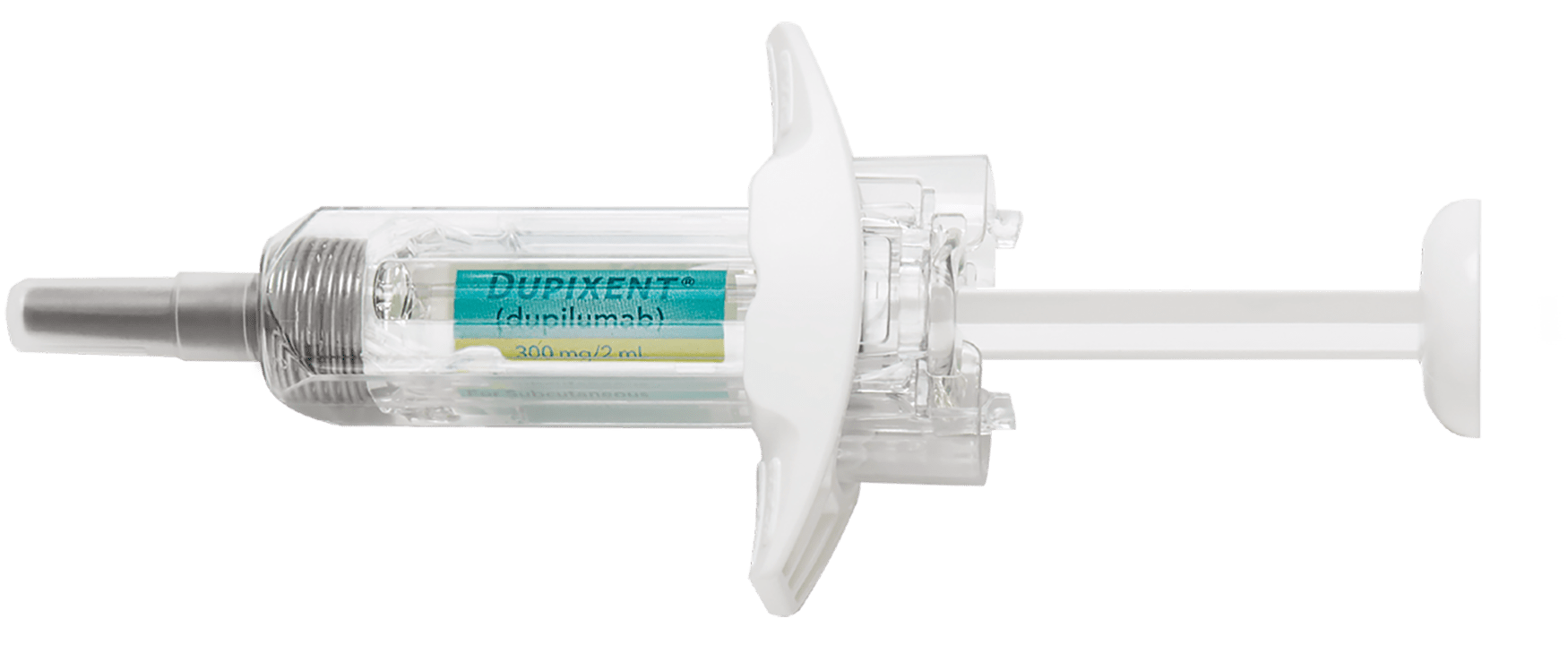
Pre-filled Syringe
- Subcutaneous injection
- Needle shield
- Finger grip for comfort
- Visual feedback
- Portable
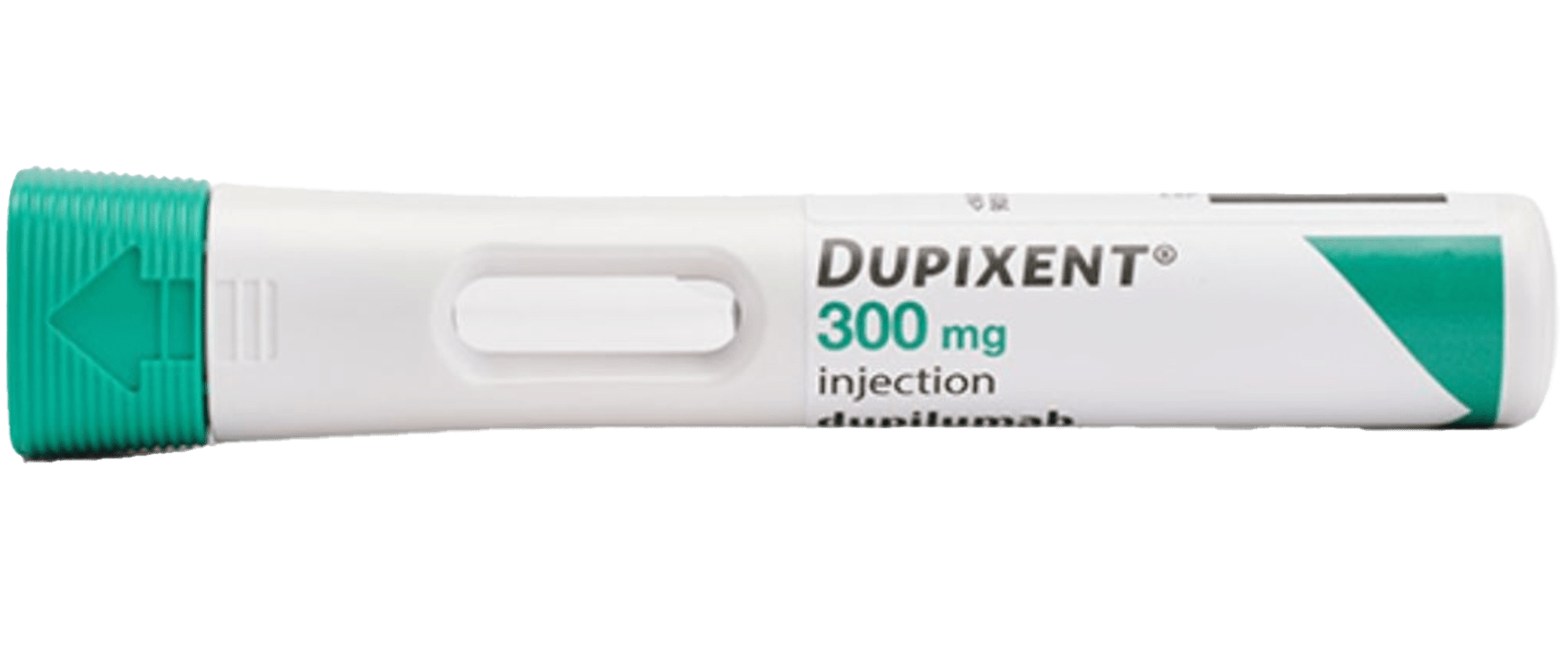
Pre-filled Pen
- Autoinjector
- Hidden needle
- 2-step safety control feature
- Visual and audible feedback
- Portable
DUPIXENT is intended for use under the guidance of a healthcare professional. A patient may self-inject DUPIXENT after training in subcutaneous injection technique using the pre-filled syringe or the pre‑filled pen. Provide proper training to patients and/or caregivers on the preparation and administration of DUPIXENT prior to use according to the Instructions for Use.1
DUPIXENT can be administered in the practice under the guidance of a healthcare professional if the patient is not an appropriate candidate for self-administration.1
DUPIXENT is sterile and preservative-free. Discard any unused portion. Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. If necessary, DUPIXENT may be kept at room temperature up to 25°C (77°F) for a maximum of 14 days. Do not store above 25°C (77°F). After removal from the refrigerator, DUPIXENT must be used within 14 days or discarded. Do not expose DUPIXENT to heat or direct sunlight. Do NOT freeze. Do NOT shake.1
a300 mg/2 mL solution.
ABBREVIATION
CRSwNP, chronic rhinosinusitis with nasal polyps
REFERENCES
- DUPIXENT (dupilumab). Summary of Product Characteristics. (UK).
- Dupixent Patient Information Leaflet. (UK).