Efficacy and burden data
DUPIXENT was studied in a clinical trial programme in severe CRSwNP1,2
| SINUS-24 (N=276) 24 Weeks1,2 | SINUS-52 (N=448) 52 Weeks1,2 | |
| Randomised, Phase 3 trial |
DUPIXENT + intranasal corticosteroids (INCS) 300 mg every 2 weeks (Q2W) for 24 weeks (n=143)
Placebo + INCS for 24 weeks (n=133) |
DUPIXENT + INCS 300 mg Q2W for 52 weeks (n=150)a
DUPIXENT + INCS 300 mg Q2W for 24 weeks, followed by every 4 weeks (Q4W) through Week 52 (n=145)b Placebo + INCS for 52 weeks (n=153) |
| Study population |
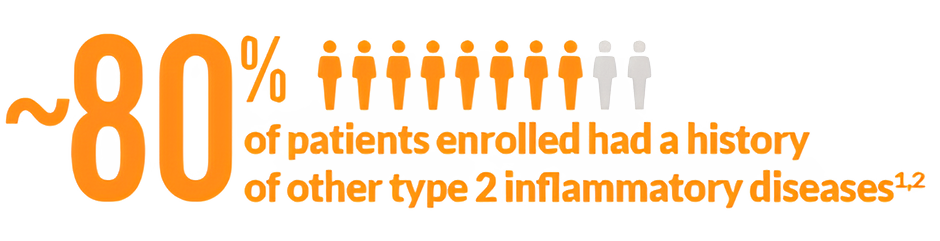
>Adults (≥18 years) on background INCS with CRSwNP despite prior sinonasal surgery or prior treatment with, or who were ineligible to receive or were intolerant to, systemic steroids in the past 2 yearsc >Patients with chronic rhinosinusitis without nasal polyps were not included in these trials >Rescue with systemic steroids or surgery was allowed at investigators’ discretion >The total population of patients in SINUS-24 and SINUS-52 was unrestricted by minimum baseline blood eosinophil count | |
| Co-primary endpoint(s) |
Least-squares-mean (LSM) change from baseline at Week 24 in:
| |
| Key shared secondary endpoint(s)a |
LSM change from baseline at Week 24 (and Week 52 for SINUS-52) in:
| |
| Prespecified endpointa |
| |
SINUS-24 Selected Baseline Demographics: Male: 57%; mean NC score (SD), range 0–3: 2.4 (0.6); mean CRSwNP duration (SD): 11 (9) years; patients with >1 prior surgery: 72%; patients with systemic steroid use in previous 2 years: 65%; mean bilateral endoscopic NPS (SD), range 0–8: 5.8 (1.3); mean LMK sinus CT total score (SD), range 0–24: 19.0 (4.4); mean UPSIT score (SD), range 0–40: 14.6 (8.5); mean loss of smell score (AM) (SD), range 0–3: 2.7 (0.5); mean SNOT-22 total score (SD), range 0–110: 49.4 (20.2).1,2
SINUS-52 Selected Baseline Demographics: Male: 62%; mean NC score (SD), range 0–3: 2.4 (0.6); mean CRSwNP duration (SD): 11 (10) years; patients with >1 prior surgery: 58%; patients with systemic steroid use in previous 2 years: 80%; mean bilateral endoscopic NPS (SD), range 0–8: 6.1 (1.2); mean LMK sinus CT total score (SD), range 0–24: 18.0 (3.8); mean UPSIT score (SD), range 0–40: 13.6 (8.0); mean loss of smell score (AM) (SD), range 0–3: 2.8 (0.5); mean SNOT-22 total score (SD), range 0–110: 51.9 (20.9).1,2
aPatients in both DUPIXENT arms in SINUS-52 received DUPIXENT 300 mg Q2W from baseline to Week 24, therefore, data up to Week 24 were pooled from both the DUPIXENT Q2W treatment arms (n=295).2
bThe recommended dose of DUPIXENT for adult patients with CRSwNP is 300 mg given subcutaneously Q2W.1
cAll patients in the placebo and DUPIXENT arms were on a background therapy of INCS, mometasone furoate nasal spray.1,2
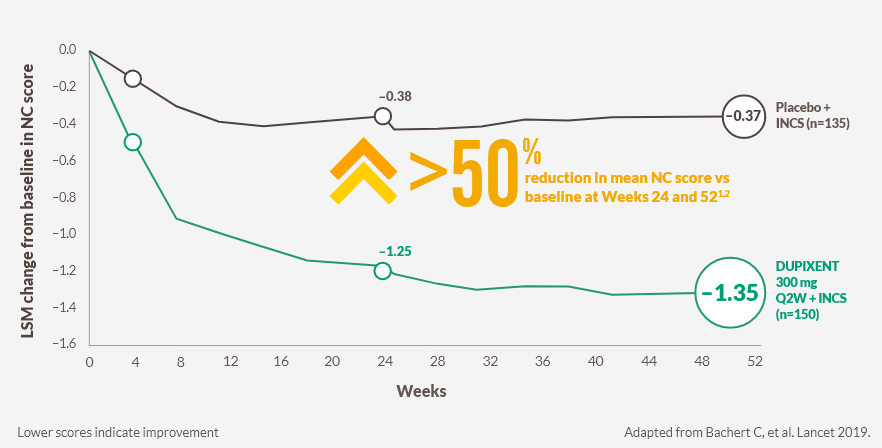
Improvement in nasal congestion sustained over 52 weeks1–4
DUPIXENT improved NC score vs placebo as early as Day 2 (post-hoc analysis),d with significant results at Week 24 (primary endpoint) and sustained through Week 52 in patients receiving INCS1–4
Change in NC score through Week 52 in SINUS-52 (secondary endpoint)1,2
CI, confidence interval; INCS, intranasal corticosteroids; LSM, least squares mean; NC, nasal congestion; Q2W, every 2 weeks
Week 24 (primary endpoint): −1.25-point improvement from a baseline score of 2.46 with DUPIXENT (n=295, pooled DUPIXENT armse) vs −0.38-point improvement from a baseline score of 2.38 with placebo (n=153; LSM difference −0.87, 95% CI −1.03 to −0.71; P<0.0001)1,2
Week 52 (secondary endpoint): −1.35‑point improvement from a baseline score of 2.48 with DUPIXENT (n=150) vs −0.37-point improvement from a baseline score of 2.38 with placebo (n=153; −0.98, 95% CI −1.17 to −0.79; P<0.0001)1,2
dPooled SINUS-24/SINUS-52 Day 1 LSM difference between DUPIXENT (n=438) and placebo (n=286): –0.07 (95% CI –0.13 to –0.01; nominal P<0.05).4
eBoth DUPIXENT arms in SINUS-52 received DUPIXENT 300 mg Q2W from baseline to Week 24.2
Underlying type 2 inflammation can drive congestion and reduce quality of life5
Nasal congestion drives many physical, social, and psychological health-related quality of life (HRQoL) burdens related to CRSwNP5
| Impacts of nasal congestion on HRQoL5 | |
| Depression Self-consciousness Loss of confidence Inability to speak up clearly |
Poor or disrupted sleep
|

Many patients reported frustration with the management of their symptoms5
NC score is a patient-reported scale rating the severity of nasal blockage, from 0 = no symptoms to 3 = severe symptoms.2
The first biologic to report a >2‑point NPS improvement in Phase 3 trials1,6,7
DUPIXENT significantly reduced the size of nasal polyps vs placebo, as measured by bilateral endoscopic NPS, as early as Week 4 (post-hoc analysis)f and sustained through Week 52, in patients receiving INCS1–3
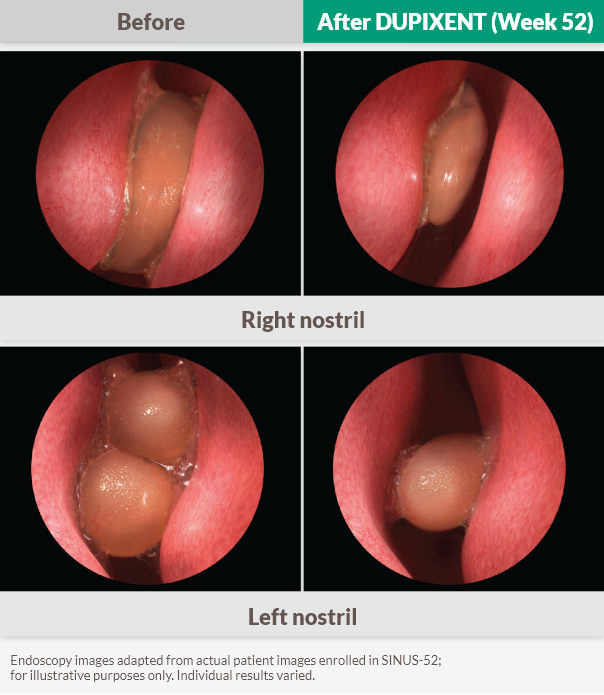

37% improvement vs baseline at Week 52 (SINUS-52 secondary endpoint)1
Week 24 (primary endpoint): −1.71-point improvement in NPS from a baseline score of 6.18 with DUPIXENT (n=295, pooled DUPIXENT arms) vs 0.10-point worsening from a baseline score of 5.96 with placebo (n=153; LSM difference −1.80, 95% CI −2.10 to −1.51; P<0.0001)1,2
Week 52 (secondary endpoint): −2.24-point improvement in NPS from a baseline score of 6.07 with DUPIXENT (n=150) vs 0.15-point worsening from a baseline score of 5.96 with placebo (n=153; LSM difference −2.40, 95% CI −2.77 to −2.02; P<0.0001)1,2
fSINUS-52 Week 4 LSM difference between DUPIXENT (n=295, pooled DUPIXENT arms) and placebo (n=153): −1.15 (95% CI, −1.40 to −0.91; nominal P<0.0001).3
Both DUPIXENT arms in SINUS-52 received DUPIXENT 300 mg Q2W from baseline to Week 24.2
Nasal polyps are a manifestation of severe chronic rhinosinusitis – Type 2 inflammation is an underlying cause8,9
Bilateral endoscopic NPS can help measure polyp formation and recurrence, which are key signs of type 2 inflammation10
NPS improvement >2 indicates substantial relief of obstruction10,11
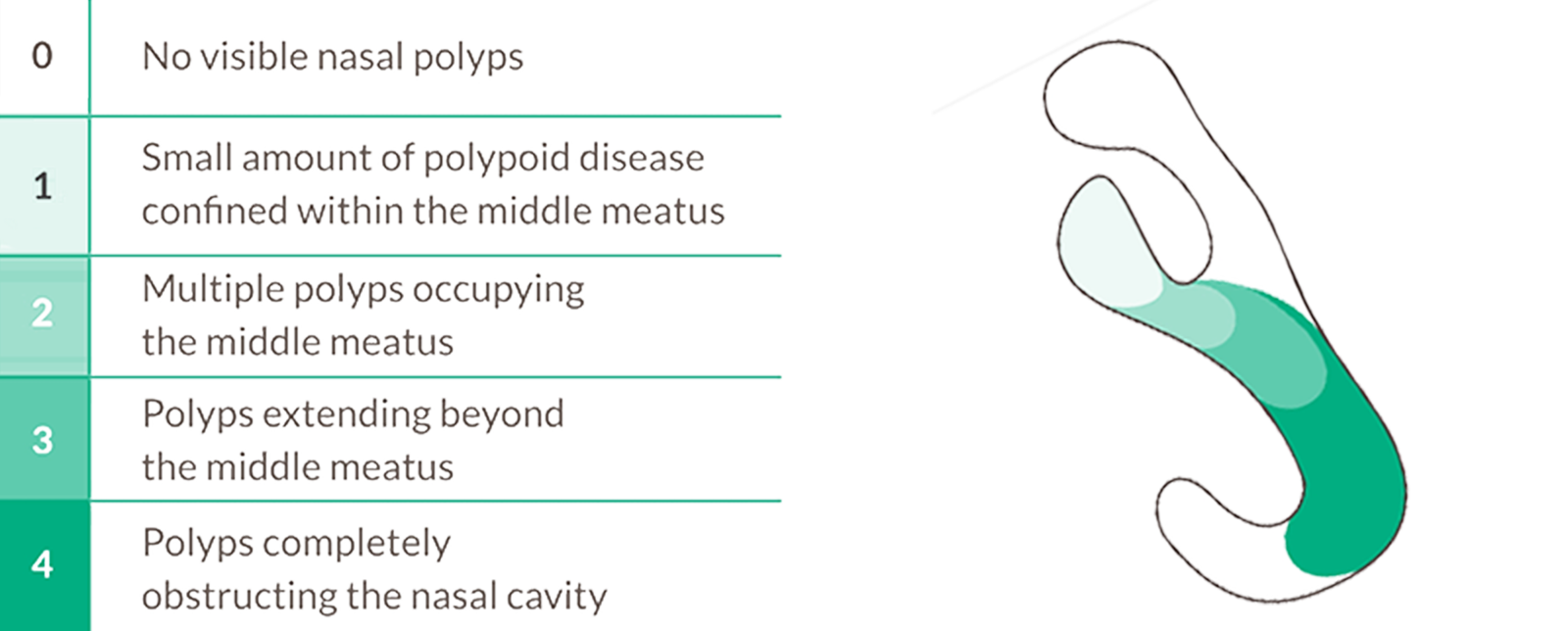

Target type 2 inflammation to reduce nasal polyp size12
Bilateral endoscopic NPS is a grading system used during nasal endoscopy to quantify the size and extent of nasal polyps in each nasal cavity. Each cavity is scored from 0 (no polyps) to 4 (polyps completely obstructing the nasal cavity). The bilateral score is the sum of both sides, ranging from 0 to 8.10
Patients regained smell as early as Day 3 (post-hoc analysis)g,3
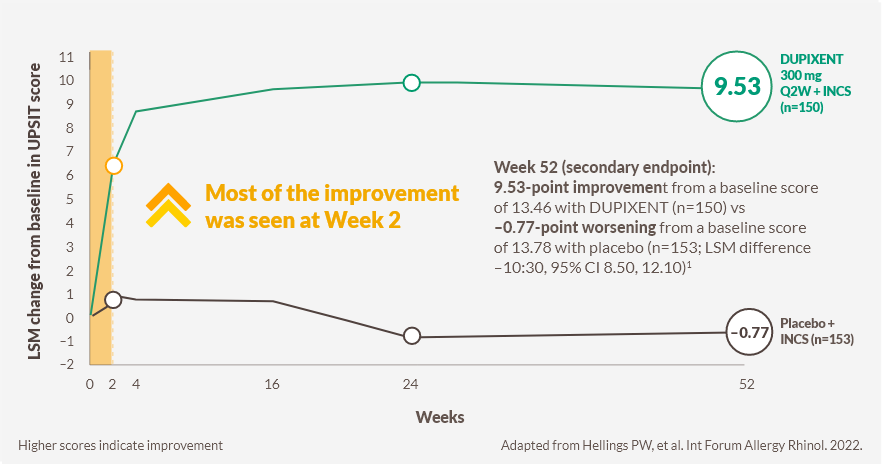
DUPIXENT improved loss of smell score (LoS) as early as Day 3g (post-hoc analysis) vs placebo, and improved sense of smell, as measured by UPSIT score, from Week 2h (post-hoc analysis) sustained through Week 52, in patients receiving INCS1–4
Change in UPSIT score through Week 52 in SINUS-52 (secondary endpoint)3
CI, confidence interval; INCS, intranasal corticosteroids; LSM, least squares mean; Q2W, every 2 weeks; UPSIT, University of Pennsylvania Smell Identification Test
LoS is a patient‑collected assessment of the daily symptom severity of decreased or loss of smell, from (0 no symptoms) to 3 (severe symptoms).4
UPSIT is a standardised 40-item scratch-and-sniff test in which patients identify odours from multiple-choice options, yielding a score from 0 to 40.4

2 out of 3 patients treated with DUPIXENT had improved sense of smell at week 24 (n=295, pooled DUPIXENT armsi)1,2
SINUS-52: 79.4% (n=228/287) of patients in the pooled DUPIXENT arms had anosmia (UPSIT score ≤18) at baseline, which was reduced to 30.0% (n=84/280) at Week 24, with almost no change observed in the placebo group (76.7% [n=115/150] at baseline vs 76.6% [n=111/145] at Week 24).2
gPooled SINUS-24/SINUS-52 Day 3: LSM difference between DUPIXENT (n=438) and placebo (n=286); –0.07 (95% difference CI –0.12 to –0.02; nominal P<0.01).4
hPooled SINUS-24/SINUS-52 Week 2: DUPIXENT (n=438) significantly improved UPSIT scores from baseline by an LSM difference of 6.81 (95% CI 5.90 to 7.73) vs 1.28 (95% CI 0.23 to 2.33) in the placebo group (n=286) (nominal P<0.001).4
iBoth DUPIXENT arms in SINUS-52 received DUPIXENT 300 mg Q2W from baseline to Week 24.2
Smell loss is one of the most important symptoms for patients2,14,15
~9 out of 10 patients who have CRSwNP experience loss of smell, which is correlated with increased disease severity and may be the first sign in the cycle of disease recurrence16,17
Smell loss is strongly associated with type 2 inflammation9,16,17

Inhibiting drivers of type 2 inflammation may play a role in improving sense of smell8,16,18
Less surgery for a majority of patients2
In a prespecified pooled analysis, DUPIXENT significantly reduced the need for sinus surgery vs placebo over 52 weeks in patients receiving INCS2

83% reduction in the need for initial or repeat sinus surgery vs placebo1,2
1.1% required rescue nasal polyp surgery with DUPIXENT (pooled DUPIXENT Q2W arms, n=438) vs 7.7% with placebo (pooled placebo, n=286) (hazard ratio [HR] 0.174; 95% CI 0.066 to 0.462)i,1,2
Surgery may still leave patients in a cycle of recurrence15,19
Despite addressing polyp obstruction, surgery does not address the underlying type 2 inflammation, which is associated with the disease burden of CRSwNP, including new polyp formation15,19
| Burdens and limitations of surgery | ||
| With each surgery, the risk of revision surgery increases and the duration between surgeries decreases20,21 | Sinus surgery does not reliably address smell loss22 | 35% polyp recurrence rate within 6 months of surgery, increasing over time19 |

EPOS and EUFOREA recommend the reduced need for surgery as a criterion of biologic response23
EPOS, European Position Paper on Rhinosinusitis and Nasal Polyps 2020; EUFOREA, European Forum for Research and Education in Allergy and Airway
DUPIXENT targets type 2 inflammation to reduce the need for systemic steroids2
In a prespecified pooled analysis, DUPIXENT reduced the need for systemic steroids vs placebo over 52 weeks in patients receiving INCS2

74% reduction in the need for systemic corticosteroid use vs placebo1,2
9.4% required rescue systemic corticosteroids with DUPIXENT (pooled DUPIXENT Q2W arms, n=438) vs 30.8% with placebo (pooled placebo, n=286) (HR 0.261; 95% Cl 0.179 to 0.379)i,1,2
Cumulative steroid use can add to the burdensome cycle patients face24
Systemic corticosteroid treatment broadly targets inflammation, and can achieve short-term symptom improvement. However, even short‑term use carries a risk of serious short- and long-term side effects24
| Limitations of OCS use25 |
| Oral corticosteroids are recommended in the short-term management of CRSwNP. Longer-term or frequent use of corticosteroids for CRSwNP is not supported by the literature and carries an increased risk of harm to the patient |
ICAR-RS, International Consensus Statement on Allergy and Rhinology: Rhinosinusitis

EPOS and EUFOREA consider a reduced need for OCS as a criterion of biologic response23
EPOS, European Position Paper on Rhinosinusitis and Nasal Polyps 2020; EUFOREA, European Forum for Research and Education in Allergy and Airway
iFor the proportion of patients requiring systemic corticosteroids or nasal polyp surgery (actual or planned) during the treatment period, the entire DUPIXENT Q2W treatment period was pooled (group A up to 52 weeks and B up to 24 weeks from SINUS-52 and the DUPIXENT group from SINUS-24) from both studies, and a forced censor was included for the SINUS-52 group B at week 24. The entire placebo treatment period, of up to 52 weeks for SINUS-52 and up to 24 weeks for SINUS-24, was pooled as well.1,2
Patients reported improved symptoms and HRQoL1,2
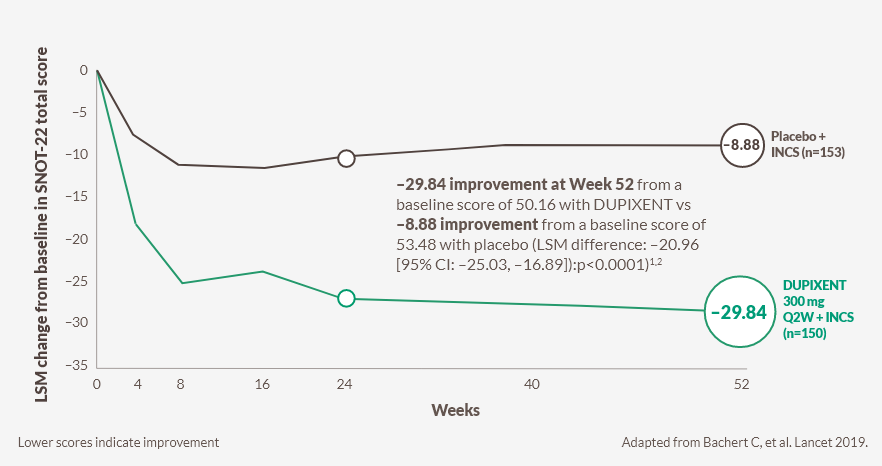
DUPIXENT significantly decreased SNOT-22 scores vs baseline at Week 24k sustained through Week 52, in patients receiving INCS2
Change in SNOT‑22 score through Week 52 in SINUS-52 (secondary endpoint)2
The maximum SNOT‑22 score is 110.26
The meaningful clinically important difference is 8.9. Lower scores indicate better health‑related quality of life.27
CI, confidence interval; INCS, intranasal corticosteroids; LSM, least squares mean; Q2W, every 2 weeks; SNOT ‑22, 22 ‑item Sinonasal Outcome Test
DUPIXENT significantly decreased SNOT-22 score vs placebo as early as Week 8 (post-hoc analysis)J,2
jPooled SINUS‑24/SINUS‑52 Week 8 (post-hoc analysis): DUPIXENT (n=438) significantly improved SNOT‑22 scores vs placebo by an LSM difference of –14.8 (95% CI –17.4 to –12.2: P<0.0001).3
kWeek 24 ( SINUS‑52): –27.77 improvement from a baseline score of 50.16 with DUPIXENT vs –10.40 improvement from a baseline score of 53.46 with placebo (LSM difference: –17.36 [95% CI –20.87 to –13.85]: p<0.0001).1,2

DUPIXENT improved a composite score, including physical symptoms, sleep and fatigue issues, and psychosocial limitations2
SNOT-22 questionnaire is a patient‑reported assessment of 22 symptoms and consequences of CRSwNP:26
1. Need to blow nose
2. Nasal blockage
3. Sneezing
4. Runny nose
5. Cough
6. Post-nasal discharge
7. Thick nasal discharge
8. Decreased sense of smell/taste
9. Ear fullness
10. Dizziness
11. Ear pain
12. Facial pain/pressure
13. Difficulty falling asleep
14. Wake up at night
15. Lack of a good night’s sleep
16. Wake up tired
17. Fatigue
18. Reduced productivity
19. Reduced concentration
20. Frustrated/restless/irritable
21. Sad
22. Embarrassed
ABBREVIATIONS
ACQ-6, six-item Asthma Control Questionnaire; CI, confidence interval; CRSwNP, chronic rhinosinusitis with nasal polyps; EPOS, European Position Paper on Rhinosinusitis and Nasal Polyps 2020; EUFOREA, European Forum for Research and Education in Allergy and Airway; FEV1, forced expiratory volume in 1 second; HRQoL, health‑related quality of life; ICAR‑RS, International Consensus Statement on Allergy and Rhinology; IL‑4/13, interleukin‑4/13; INCS, intranasal corticosteroids; LoS, loss of smell score; LSM, least squares mean; LMK‑CT, Lund‑Mackay computed tomography; NC, nasal congestion/obstruction; NP, nasal polyps; NPS, nasal polyp score; OCS, oral corticosteroids; OSN, olfactory sensory neurons; Q2/4W, every 2/4 weeks; SD, standard deviation; SNOT‑22, 22‑item Sinonasal Outcome Test; UPSIT, University of Pennsylvania Smell Identification Test
REFERENCES
- DUPIXENT (dupilumab) Summary of Product Characteristics (UK).
- Bachert C, et al. Lancet. 2019;394(10209):1638‑50.
- Hellings PW, et al. Int Forum Allergy Rhinol. 2022;12(7):958‑62.
- Canonica GW, et al. J Allergy Clin Immunol Pract. 2022;10(6):1515‑26.
- Bachert C, et al. J Asthma Allergy. 2021;14127‑34.
- XOLAIR (omalizumab). Summary of Product Characteristics.
- NUCALA (mepolizumab). Summary of Product Characteristics.
- Gandhi NA, et al. Nat Rev Drug Discov. 2016;15(1):35‑50.
- Maspero J, et al. ERJ Open Res. 2022;8(3):00576‑2021.
- Han JK, et al. Laryngoscope. 2022;132(2):265-271.
- Ferguson BJ, et al. Categorization of nasal polyps. In: Önerci TM, J. FB, editors,eds. Nasal Polyposis: Springer‑Verlag; 2010. p. 103‑10.
- Bachert C, et al. J Allergy Clin Immunol. 2020;145(3):725‑39.
- Sanofi. Data on File. SUPP‑2202664. 10 November 2022.
- Chung JH, et al. Ann Otol Rhinol Laryngol. 2015;124(8):663‑70.
- De Corso E et al. Acta Otolaryn. 2023;43(suppl 1):S3–S13.
- Yan X, et al. Laryngoscope Investig Otolaryngol. 2020;5(6):992‑1002.
- Macchi A, et al. Front Allergy. 2023;41083964.
- Mullol J, et al. J Allergy Clin Immunol. 2020;145(3):773‑76.
- DeConde AS, et al. Laryngoscope. 2017;127(3):550‑55.
- Smith KA, et al. Int Forum Allergy Rhinol. 2019;9(4):402‑08.
- Hopkins C, Lund V. Rhinology. 2021;59(3):277‑83.
- Lourijsen ES, et al. Lancet Respir Med. 2022;10(4):337‑46.
- Fokkens WJ, et al. Rhinology. 2023;61(3):194‑202.
- Hox V, et al. Clin Transl Allergy. 2020;101.
- Orlandi RR, et al. Int Forum Allergy Rhinol. 2016;6 Suppl 1S22‑209.
- Khan AH, et al. Laryngoscope. 2022;132(5):933‑41.
- Hopkins C, et al. Clin Otolaryngol. 2009;34(5):447–54.
- Juniper EF et al. Respir Med. 2005;99:553–58.