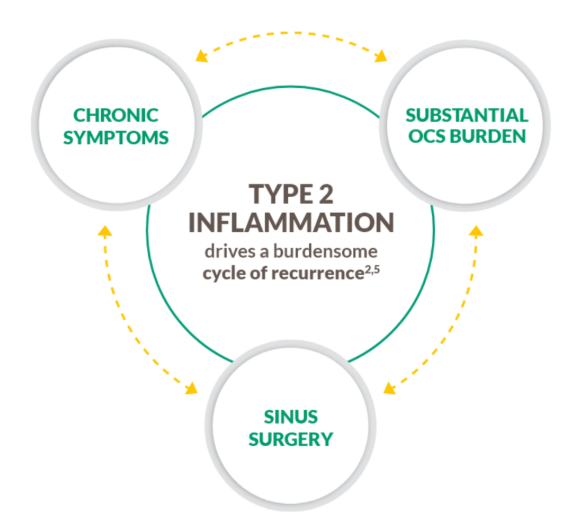
Type 2 inflammation
Unmet need–Cycle of Recurrence
Severe CRSwNP is not just an obstructive disease.
Severe CRSwNP is typically a chronic type 2 inflammatory disease.1–4
~9 out of 10 patients with CRSwNP have type 2 inflammation, an underlying driver of complications, including polyp formation.2–5
OCS, oral corticosteroids
Help break the cycle with a treatment that targets type 2 inflammation2,4,5
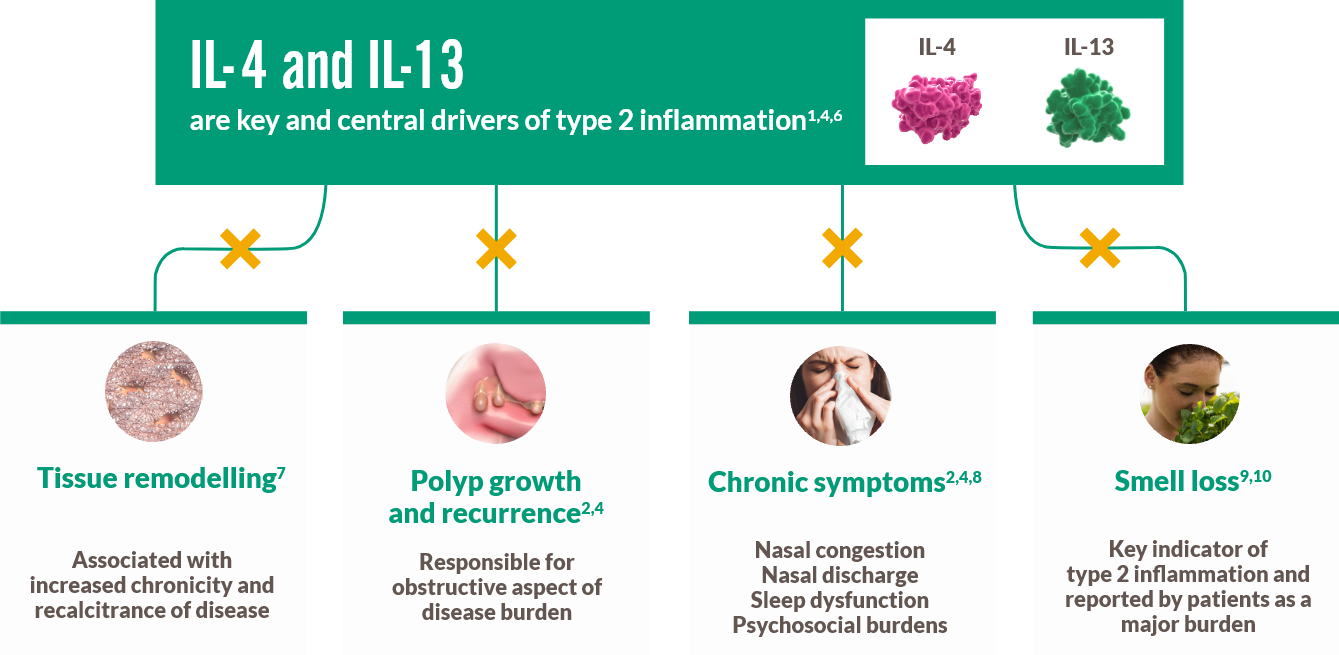
Mechanism of Action
DUPIXENT centrally targets underlying type 2 inflammation by inhibiting key drivers IL‑4 and IL‑13 in severe CRSwNP1,4,6
ABBREVIATIONS
CRSwNP, chronic rhinosinusitis with nasal polyps; IL‑4/13, interleukin‑4/13; OCS, oral corticosteroids
REFERENCES
- Schleimer RP. Annu Rev Pathol. 2017;12:331‑57.
- Maspero J, et al. ERJ Open Res. 2022;8(3).
- Gandhi NA, et al. Nat Rev Drug Discov. 2016;15(1):35‑50.
- De Corso E, et al. J Pers Med. 2022;12(8):1251.
- Stevens WW, et al. J Allergy Clin Immunol Pract. 2019;7:2812-2820.
- DUPIXENT (dupilumab) Summary of Product Characteristics (UK).
- Amirapu S, et al. Int J Otolaryngol. 2021;2021:7428955.
- Orlandi RR, et al. Int Forum Allergy Rhinol. 2021;11:213–739.
- Macchi A, et al. Front Allergy. 2023;4:1083964.
- Yan X, et al. Laryngoscope Investig Otolaryngol. 2020;5(6):992‑1002.