- Article
- Source: Campus Sanofi
- 12 Nov 2025
The Role of Mucus Plugging in Asthma and Its Clinical Burden


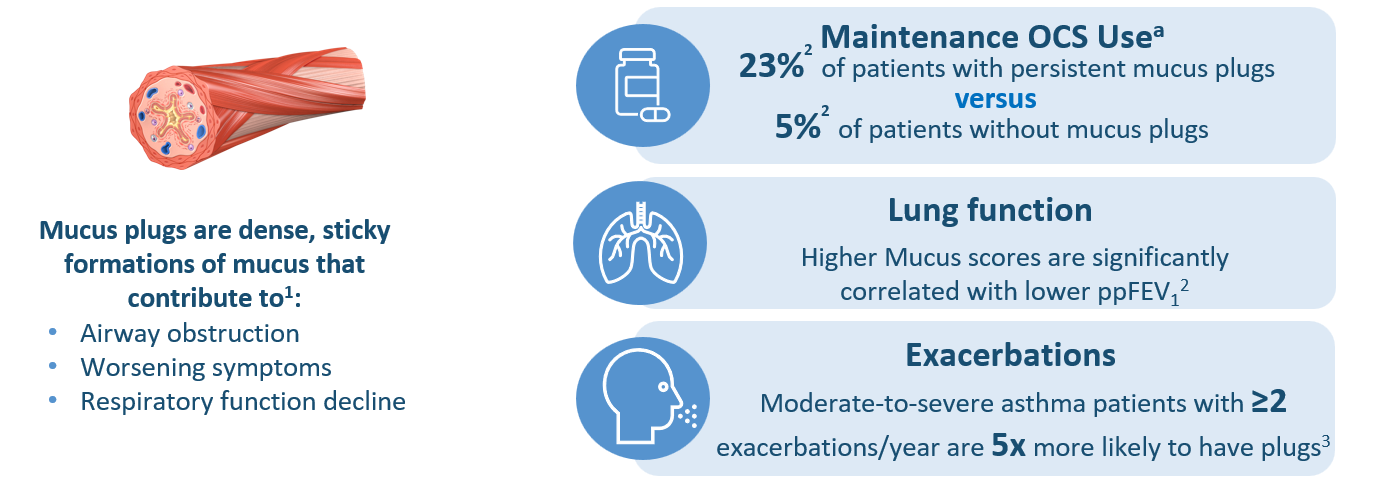

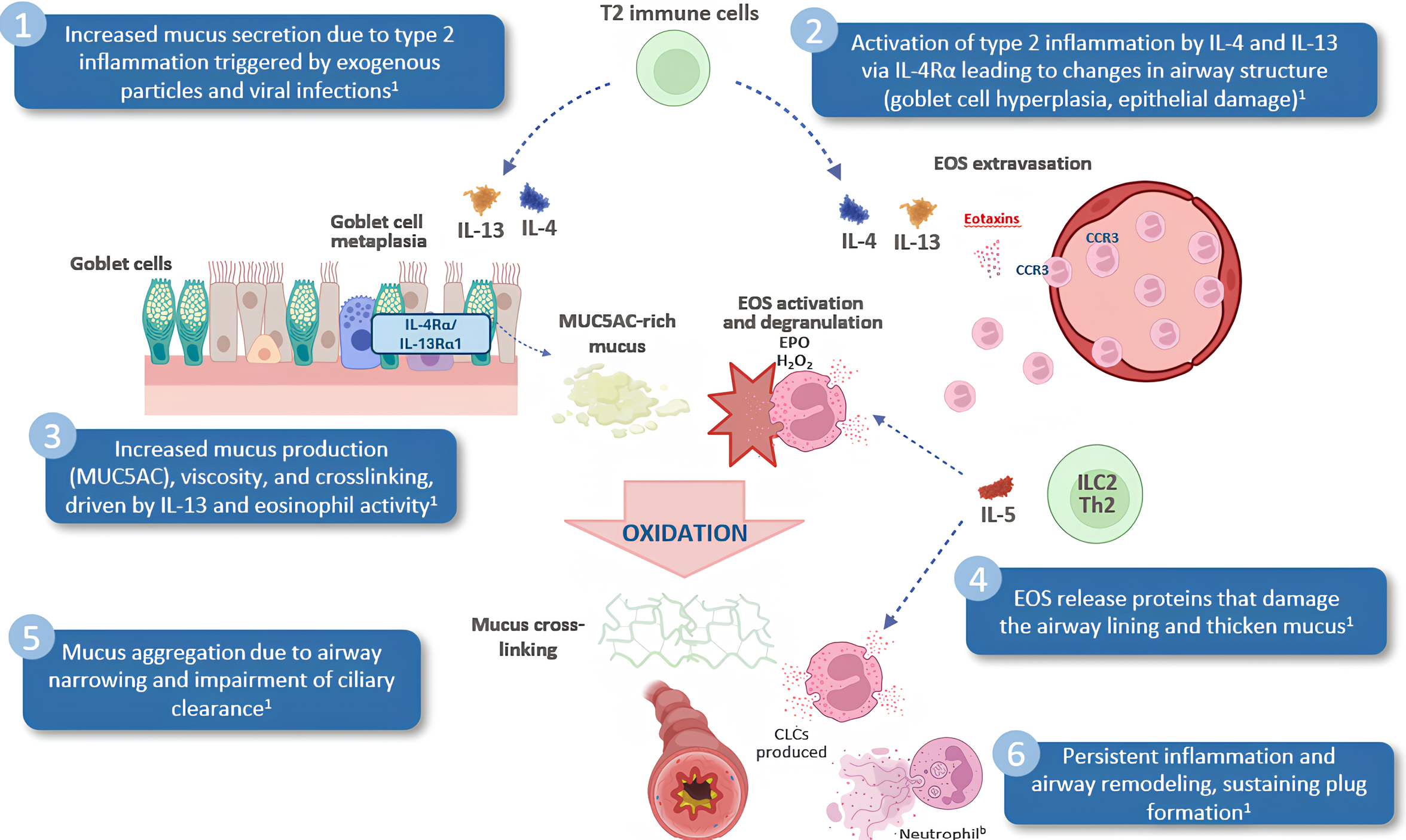

Figure created with BioRender.com. aDaily OCS use bIn patients with asthma, mucus plugging is primarily driven by type 2 inflammation; however, type 1 immune responses, including neutrophil involvement, may also contribute.1
CLC, Charcot–Leyden crystal; EC, epithelial cell; eDNA, extracellular DNA; EOS, eosinophils; EPO, eosinophil peroxidase; H2O2, hydrogen peroxide; IL, interleukin; ILC, innate lymphoid cell; MUC5AC, mucin 5AC; OCS, oral corticosteroids; ppFEV1, percent predicted forced expiratory volume in 1 second; Th, T-helper.
1. Aegerter H, Lambrecht BN. Annu Rev Pathol. 2023;18:387–409. 2. Tang M, et al. Am J Respir Crit Care Med. 2022;205:1036–1045. 3. Chan R, et al. Allergy Clin Immunol Pract. 2023;11:195–199. 4. Audousset C, et al. Respiratory Res. 2024;25:52. 5. Dunican EM, et al. Ann Am Thorac Soc. 2018;15:S184–191.

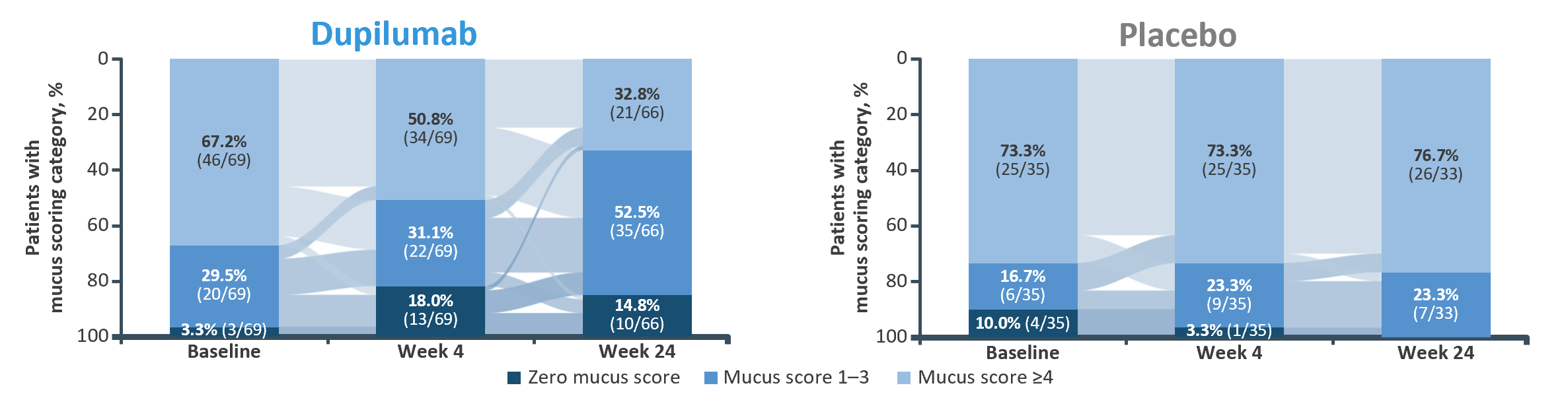
Mucus plug score has a strong negative association with lung function parameters such as FEV1 and FVC1–3
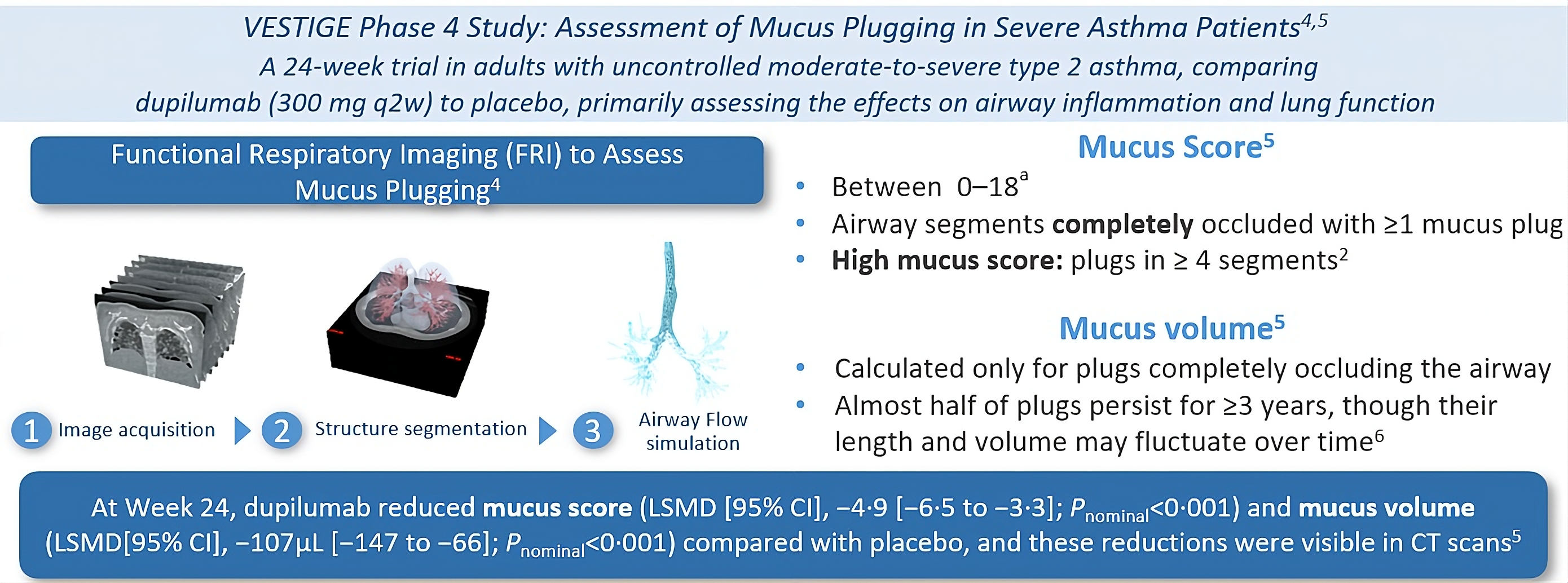
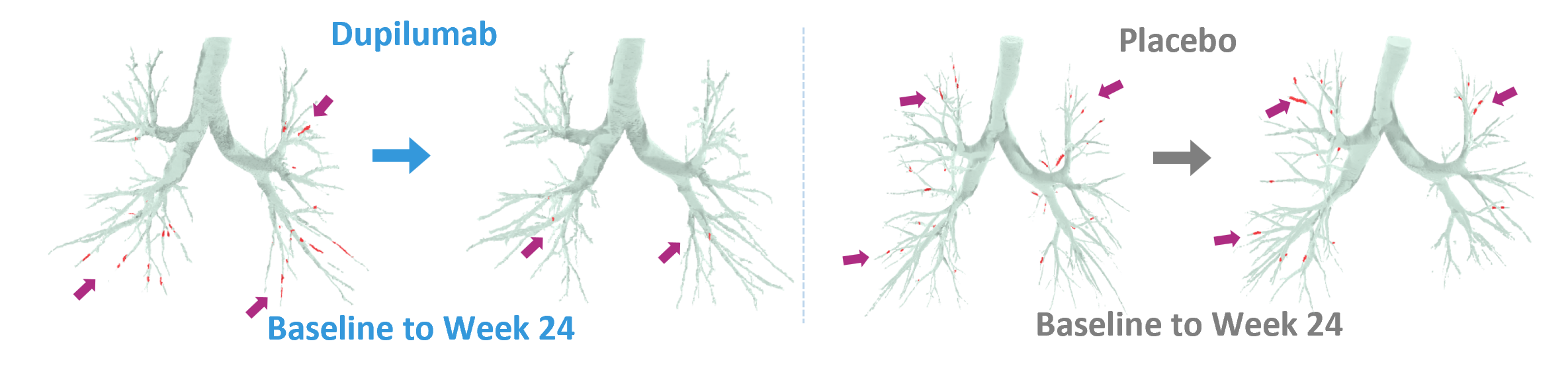


aCorresponding to the 18 bronchopulmonary segments present in most people. BD, bronchodilator; CT, computed tomography; FEV1, forced expiratory volume in 1 second; LSMD, least squares mean difference; ppFEV1, percent predicted forced expiratory volume in 1 second.
1. Jaramillo AM, et al. Allergol Int. 2024;73:375–381. 2. Dunican EM, et al. J Clin Invest. 2018;128:997–1009. 3. Tang M, et al. Am J Respir Crit Care Med. 2022;205:1036–1045. 4. https://www.fluidda.com/lung-imaging-fri/. Accessed January 2025.
5. Castro M, et al. Lancet Respir Med. 2025. In press. doi:10.1016/S2213-2600(24)00362-X. 6. Huang BK, et al. JCI Insight. 2024;9(3):e174124. 7. Porsbjerg C, et al. ERS. 2024. Presentation OA3649.
To report any side effect(s):
Saudi Arabia: National Pharmacovigilance and Drug Safety Centre (NPC)
SFDA call center: 19999. E-mail: npc.drug@sfda.gov.sa. Website: https://ade.sfda.gov.sa
Full Prescribing Information is available upon request: SANOFI, Kingdom of Saudi Arabia, P.O. Box 9874, Jeddah 21423, K.S.A.
Tel: +966-12-669-3318, Fax: +966-12-663-6191
For Medical Information, Please contact: +966-12-669-3318, ksa.medicalinformation@sanofi.com. For Pharmacovigilance, Please contact: +966-54-428-4797, ksa_pharmacovigilance@sanofi.com. To report any product technical complaints, kindly contact quality.greatergulf@sanofi.com
SANOFI, Level 3, One JLT, Jumeirah Lake Towers (JLT), DMCC, PO Box 53899, Dubai, UAE | Tel: +971 4 550 3600 | Fax: +971 4 5521050
For further Medical Information, please contact: For UAE ✆ 800 MEDICAL Toll-Free Number. For all Gulf Countries ✆+971 45 50 38 63 or email: medical-information.gulf@sanofi.com. Full Prescribing Information is available upon request. To report adverse events please call: +971 561747001 or email Gulf.Pharmacovigilance@sanofi.com
MAT-AE-2500787