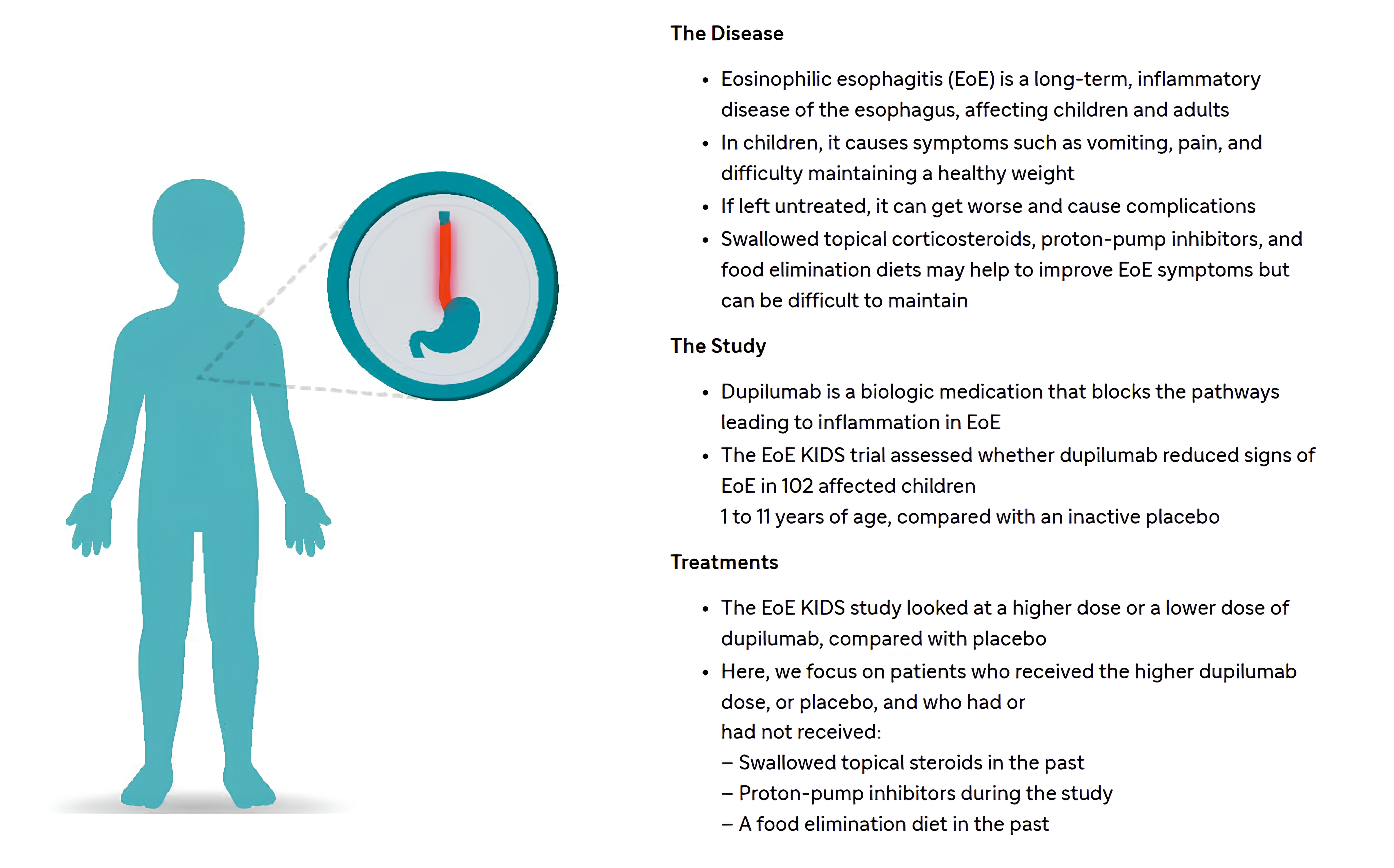
- Article
- Source: Campus Sanofi
- 10 Nov 2025
Dupilumab in Pediatric EoE: Phase 3 KIDS Study Results
Study Overview: The analysis examines dupilumab efficacy stratified by previous treatment history, including:
- Prior or current use of swallowed topical corticosteroids (STCs)
- Concomitant proton pump inhibitor (PPI) treatment
- History of food elimination diet

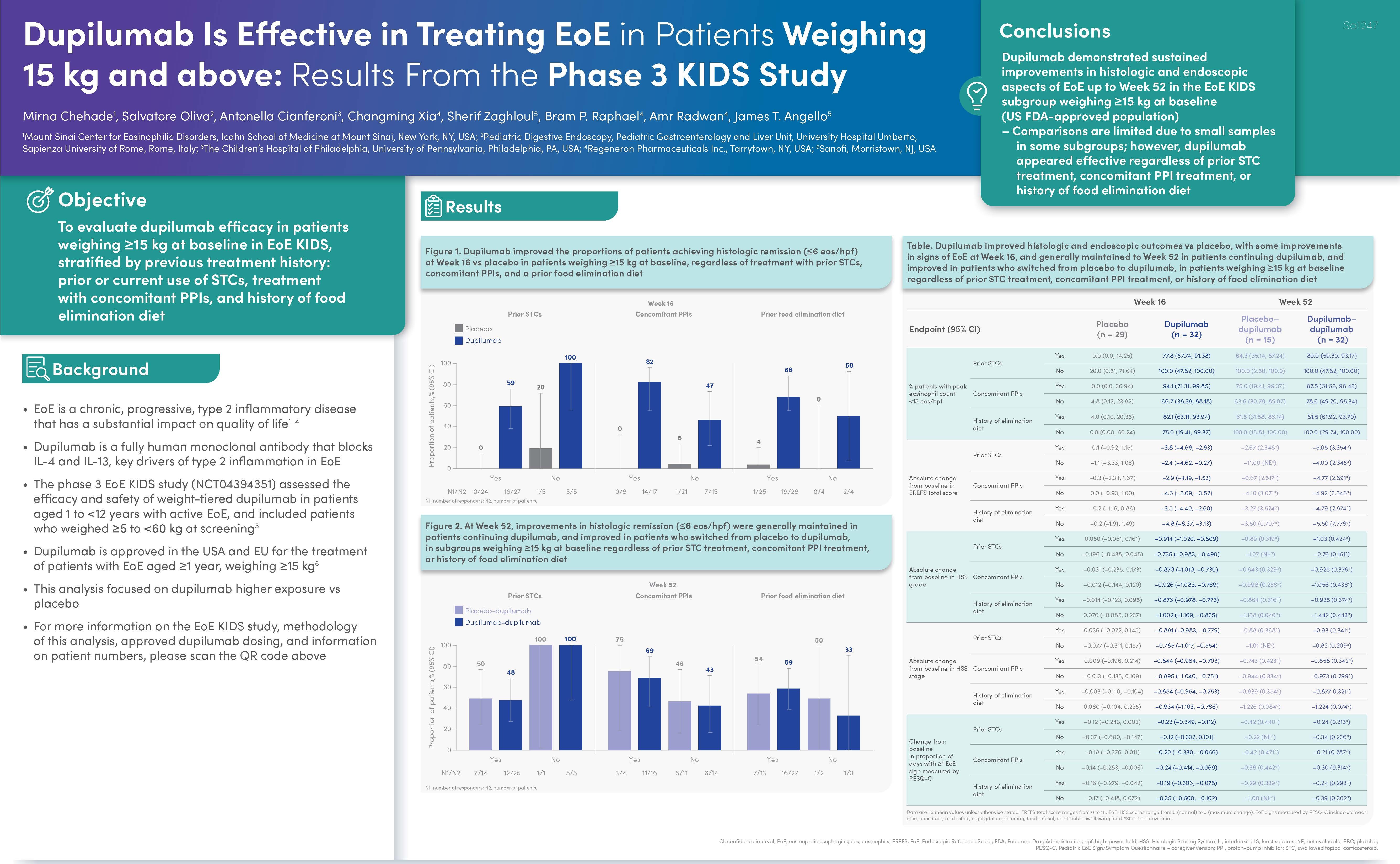
- At 16 weeks and over 52 weeks of treatment, dupilumab led to:
Chehade M, et al. Dupilumab is Effective in Treating EoE in Patients Weighing ≥15 kg: Results from the Phase 3 EoE KIDS Study. Presented at Digestive Diseases Week (DDW) 2025: San Diego, CA, USA; May 3–6, 2025.
Disclosures:Chehade M: Adare Pharma Solutions/Ellodi Pharmaceuticals, Allakos, AstraZeneca, BMS, Nexstone Immunology/Uniquity Bio, Phathom, Recludix Pharma, Regeneron Pharmaceuticals Inc., Sanofi, Shire/Takeda – consultant; Adare Pharma Solutions/Ellodi Pharmaceuticals, Allakos,
AstraZeneca, BMS, Danone, Regeneron Pharmaceuticals Inc., Shire/Takeda – research funding. Oliva S: Celgene/Receptos/BMS, Medtronic, Ocean Pharma, Sanofi/Regeneron – advisory board member; Medtronic, Sanofi/Regeneron – speaker fees. Cianferoni A: AstraZeneca – consultant;
DBV Technologies, Regeneron Pharmaceuticals Inc., Sanofi – medical advisory board member; Aimmune, DBV Technologies – grant support. Xia C, Raphael BP, Radwan A: Regeneron Pharmaceuticals Inc. – employees and shareholders. Zaghloul S, Angello JT: Sanofi – employees, may hold
stock and/or stock options in the company.
References: 1. Straumann A, Katzka DA. Gastroenterology. 2018;154:346-59. 2. Mukkada V, et al. Clin Gastroenterol Hepatol. 2018;16:495-503. 3. Stern E, et al. Dis Esophagus. 2018;31:1-7. 4. Dellon ES, Hirano I. Gastroenterology. 2018;154:319-32. 5. Chehade M, et al. N Engl J Med.
2024;390:2239-51. 6. DUPIXENT® (dupilumab). Highlights of Prescribing Information. US Food and Drug Administration. Revised September 2024. Available from: www.regeneron.com/downloads/dupixent_fpi.pdf. Accessed April 2025.
Acknowledgements and funding sources: Data fi rst presented at the 2024 American College of Gastroenterology (ACG) Annual Meeting; Philadelphia, PA, USA; October 25–30, 2024. Research sponsored by Sanofi and Regeneron Pharmaceuticals Inc. ClinicalTrials.gov Identifi er: NCT04394351.
The authors thank Ti§ any Pela, of Sanofi , and Juby A. Jacob-Nara, formerly of Sanofi , for their insights and guidance. Medical writing/editorial assistance was provided by Louise Wright, PhD, of Adelphi Communications, Bollington, UK, and was funded by Sanofi and Regeneron
Pharmaceuticals Inc., according to the Good Publication Practice guidelines.
Presented at Digestive Diseases Week (DDW) 2025: San Diego, CA, USA; May 3–6, 2025.


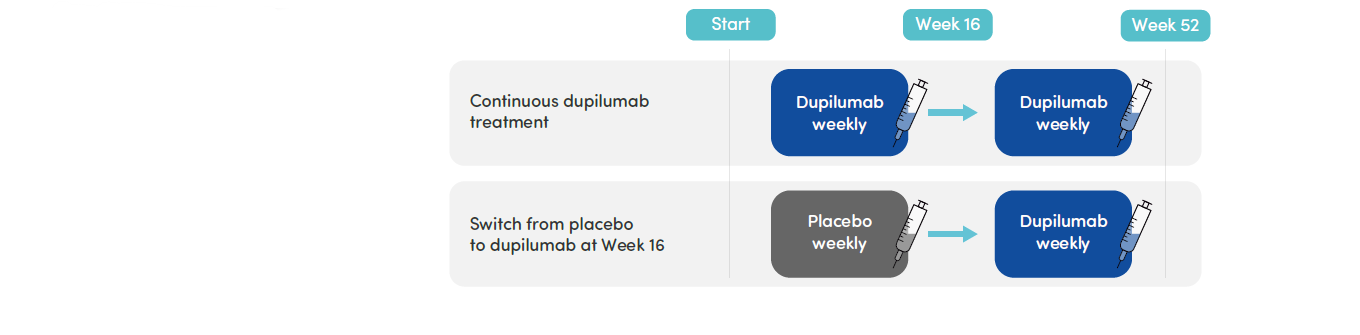
- The EoE KIDS study comprised a double-blind, placebo-controlled period (Part A) where patients were randomized 2:2:1:1 to receive weight-tiered dupilumab higher exposure or lower exposure, or placebo (2 groups) for 16 weeks, and a 36-week extension period where all patients received dupilumab higher exposure or lower exposure (Part B)
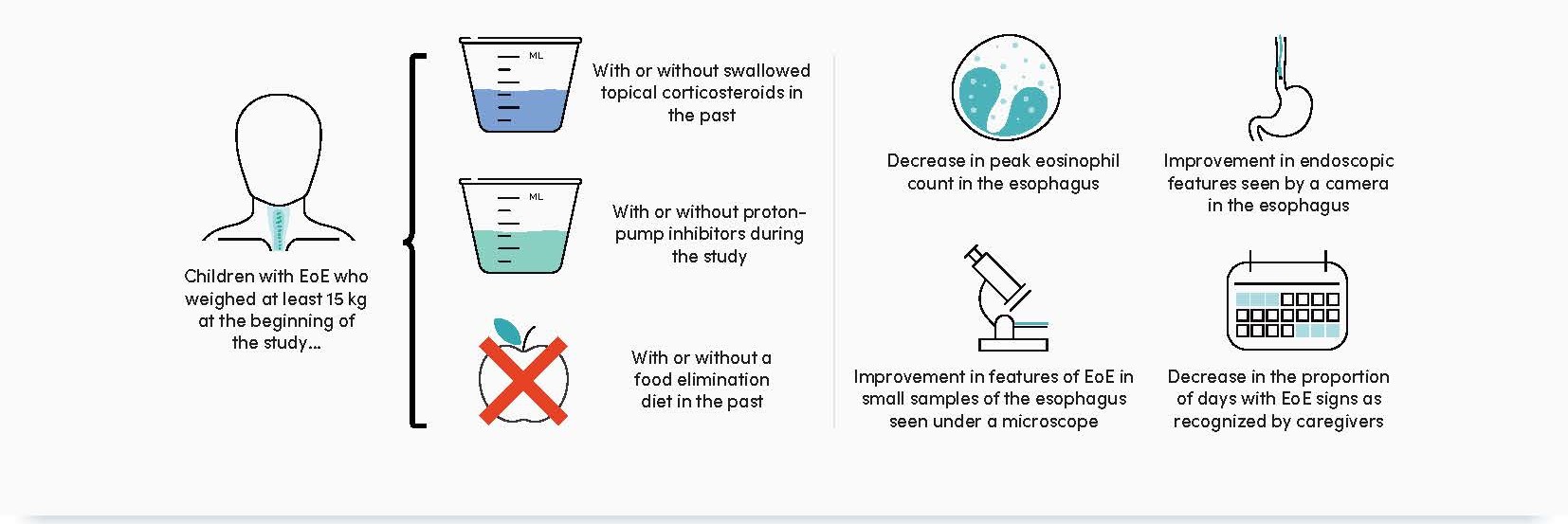
– Patients eligible to be enrolled in EoE KIDS must have weighed ≥5 to <60 kg at screening
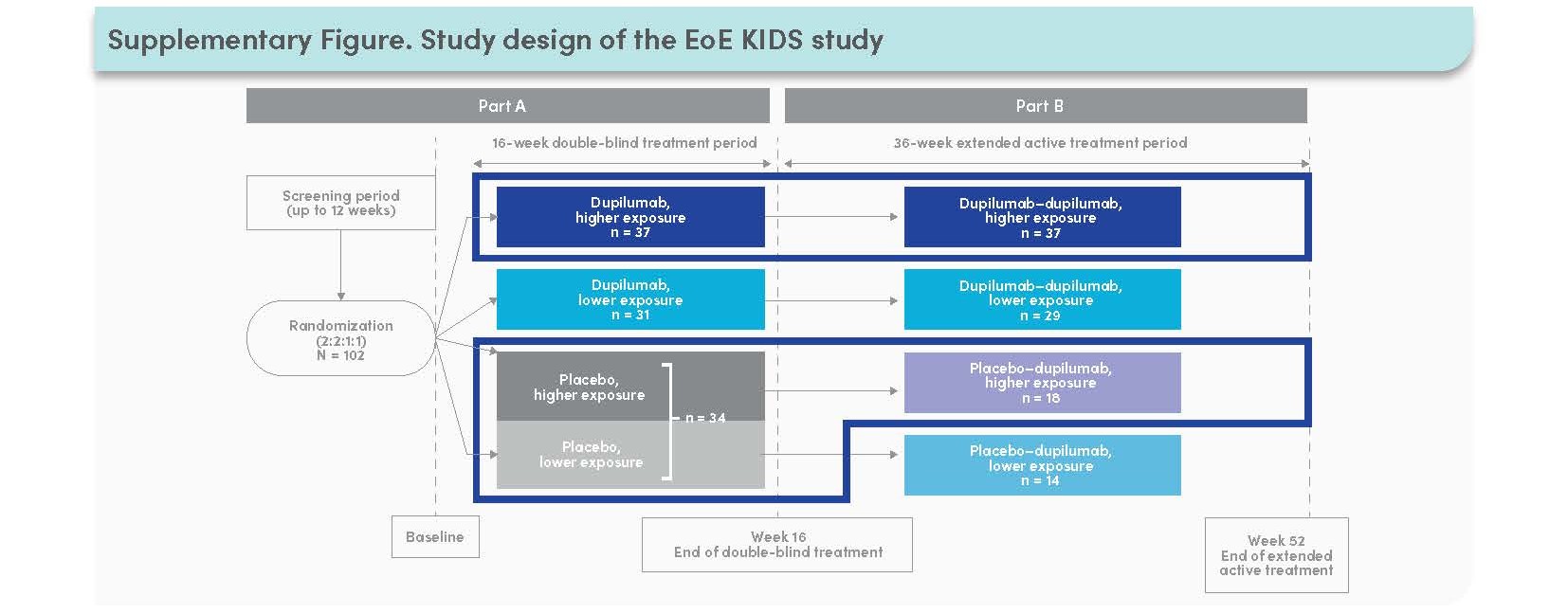
- This analysis focused on higher-exposure dupilumab vs placebo
- Patients in EoE KIDS who weighed ≥15 kg at baseline were stratified according to:
– Prior use of swallowed topical corticosteroids: yes/no
– Treatment with concomitant proton-pump inhibitors: yes/no
– History of food elimination diet: yes/no - Outcomes in these subgroups assessed at Weeks 16 and 52 were: the proportion of patients achieving peak esophageal intraepithelial eosinophil count ≤6 eos/hpf and <15 eos/hpf, and absolute change from baseline in EoE-Endoscopic Reference Score total score, EoE
Histologic Scoring System grade and stage scores, and the proportion of days with ≥1 sign of EoE (assessed by the Pediatric EoE Sign/ Symptom Questionnaire – caregiver version)


eos, eosinophils; EREFS, EoE-Endoscopic Reference Score; hpf, high-power field; HSS, Histologic Scoring System; LS, least squares; PESQ-C, Pediatric EoE Sign/Symptom Questionnaire caregiver version; PPI, proton-pump inhibitor; STC, swallowed topical corticosteroid.
MAT-AE-2500706