A monoclonal antibody (mAb) injection for the prevention of respiratory syncytial virus (RSV) disease1
Beyfortus was approved to prevent RSV lower respiratory tract disease by the US Food and Drug Administration in July of 2023 in1,2:
- Neonates and infants born during or entering their first RSV season.
- Children up to 24 months who remain vulnerable to severe RSV disease through their second RSV season.
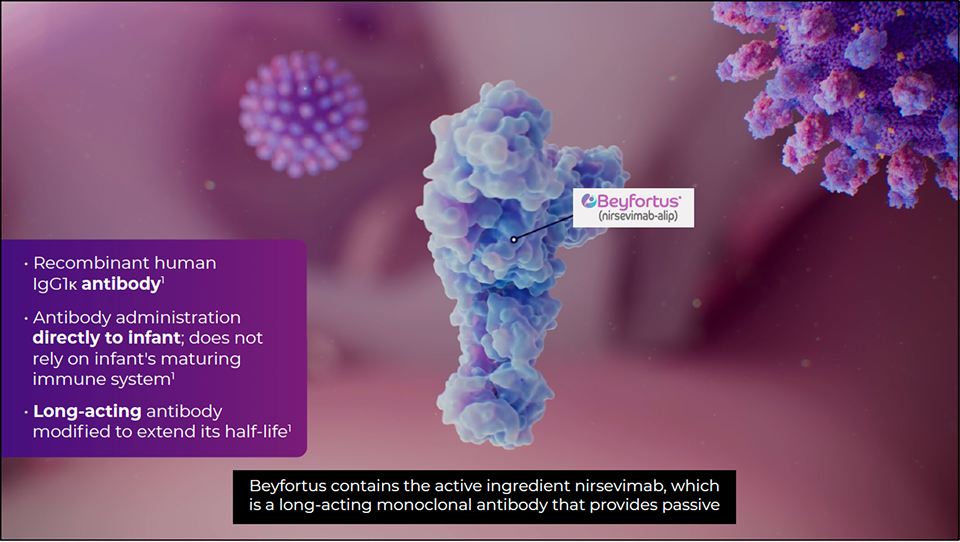
Beyfortus is a recombinant human immunoglobulin G1 kappa (IgG1κ) mAb designed to prevent RSV lower respiratory tract disease in neonates and infants.1
Unlike an infection or vaccination, RSV antibody therapy does not stimulate the immune system. Instead, mAbs provide direct protection against the disease, representing a form of passive immunization against RSV disease. Protection is typically at its peak in the weeks following administration of Beyfortus and diminishes gradually over time.3
Beyfortus protects against RSV disease by preventing membrane fusion
Beyfortus is a protein-directed fusion inhibitor that provides long-acting passive immunity to RSV disease by binding to a conserved epitope on the RSV prefusion F protein, interfering with cellular membrane fusion, and thus preventing viral entry into cells.1
The mechanism of action for Beyfortus is explained in the video below.
The role of glycoproteins in RSV
RSV is coated with two types of glycoproteins: the attachment glycoprotein (G protein) and the fusion glycoprotein (F protein). Only the F protein is essential for RSV entry into the cells lining the respiratory tract.4
The RSV F protein exists as a type I viral fusion glycoprotein that forms a trimeric complex and mediates viral entry. 5,6
Blocking viral attachment and entry into bronchial epithelial cells
Upon infection, the RSV F protein—typically responsible for facilitating pH-independent viral membrane fusion with the host cell plasma membrane—triggers the merging of adjacent cells when expressed on the cell surface.4
This membrane fusion process results in the formation of syncytia, which are large, multinucleated structures that can induce epithelial lesions in the upper respiratory tract. The formation of syncytia (cell-cell fusion) is what gives RSV its characteristic name.4,7
YTE substitution prolongs the half-life of Beyfortus RSV antibody therapy
Beyfortus RSV antibody therapy is long-acting due to a specially designed triple amino acid substitution—M252Y/S254T/T256E (YTE)—in its fragment crystallizable (Fc) region, which increases its affinity to the neonatal Fc receptor.8
This YTE substitution in the Fc segment of Beyfortus prolongs its serum half-life and is designed to provide season-long protection that extends through 5 months after injection with a single intramuscular dose.1,8
Specifically, the YTE modification results in a long anti-RSV antibody half-life extension from 21–28 days to 87–117 days.1,9
Discover more about Beyfortus
Important Safety Information
References: 1. Beyfortus (nirsevimab-alip). Prescribing Information. Sanofi. 2. US CDC Advisory Committee unanimously recommends routine use of Beyfortus™ (nirsevimab-alip) to protect infants against RSV disease. Sanofi. August 3, 2023. Accessed May 26, 2025. https://www.sanofi.com/assets/dotcom/pressreleases/2023/2023-08-03-19-21-36-2718475-en.pdf 3. RSV immunization guidance for infants and young children. Centers for Disease Control and Prevention. Updated August 30, 2024. Accessed May 5, 2025. https://www.cdc.gov/rsv/hcp/vaccine-clinical-guidance/infants-young-children.html 4. Battles MB, Langedijk JP, Furmanova-Hollenstein P, et al. Molecular mechanism of respiratory syncytial virus fusion inhibitors. Nat Chem Biol. 2016;12(2):87-93. 5. Hu M, Bogoyevitch MA, Jans DA. Impact of respiratory syncytial virus infection on host functions: implications for antiviral strategies. Physiol Rev. 2020;100(4):1527-1594. 6. Gilman MSA, Furmanova-Hollenstein P, Pascual G, et al. Transient opening of trimeric prefusion RSV F proteins. Nat Commun. 2019;10(1):2105. 7. Fraire AE, Woda BA. Respiratory syncytial virus. In: Fraire AE, Woda BA, Welsh R, Kradin R, eds. Viruses and the Lung. Springer Berlin Heidelberg; 2014:95-99. 8. Brady T, Cayatte C, Roe TL, et al. Fc-mediated functions of nirsevimab complement direct respiratory syncytial virus neutralization but are not required for optimal prophylactic protection. Front Immunol. 2023;14:1283120. 9. Domachowske J, Khan AA, Esser MT, et al. Safety, tolerability and pharmacokinetics of MEDI8897, an extended half-life single-dose respiratory syncytial virus prefusion F-targeting monoclonal antibody administered as a single dose to healthy preterm infants. Pediatr Infect Dis J. 2018;37(9):886-892.

