While SRT and ERT work in different ways, the goal of each treatment is the same: To reduce the accumulation of excess GL-1.1
Understanding treatment options
Treatment options are available to address the visceral, hematologic, and skeletal manifestations of Gaucher disease type 1 and type 3. There is no treatment available to address the CNS manifestations of Gaucher disease type 3.1-3
Treating Gaucher disease
Depending on the type of Gaucher disease, there may be up to 2 treatment approaches available.1-3
- Enzyme replacement therapy (ERT), available for non-CNS manifestations of Gaucher disease type 1 and type 3
- Substrate reduction therapy (SRT), available for Gaucher disease type 1 only
While SRT and ERT work in different ways, the goal of each treatment is the same: to reduce the accumulation of excess GL-1.1
Enzyme replacement therapy (ERT)
ERT works by replacing deficient β-glucosidase needed to break down excess GL-1.1,2
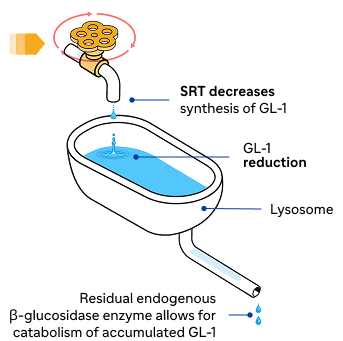
Substrate reduction therapy (SRT)
SRT inhibits glucosylceramide synthase, thereby slowing down system-wide accumulation of GL-1, allowing the cells’ residual enzyme activity to break down the substrate.1,4,5
Untreated
Deficient enzymatic catabolism of GL-1 due to Gaucher disease leads to accumulation of GL-1 in the lysosomes5
SRT
SRT specifically inhibits GCS, slowing down the production of GL-1 to reduce accumulation of GL-11,5
ERT
ERT works by replacing deficient β-glucosidase needed to break down excess GL-11,2
CNS=central nervous system.
Indication
References: 1. Stirnemann J, Belmatoug N, Camou F, et al. A review of Gaucher disease pathophysiology, clinical presentation and treatments. Int J Mol Sci. 2017;18(2):441. 2. Cerezyme (imiglucerase). Prescribing information. Genzyme Corporation, Cambridge, MA. 3. Zhong W, Li D, Fei Y, Hong P. A review of type 3 Gaucher disease: unique neurological manifestations and advances in treatment. Acta Neurol Belg. 2024;124(4):1213-1223. 4. Deegan PB, Cox TM. Imiglucerase in the treatment of Gaucher disease: a history and perspective. Drug Des Devel Ther. 2012;6:81-106. 5. Cerdelga (eliglustat). Prescribing information. Genzyme Corporation, Cambridge, MA.
