Cerezyme improved visceral and hematologic parameters.1
Pivotal, phase 3 study design and outcomes
Cerezyme was proven to be safe and effective, as shown in a pivotal, phase 3 study that studied visceral, hematologic, and bone parameters in adult and pediatric patients with Gaucher disease type 1.1
Study design1,2
At 9 months, patients were unblinded and patients were allowed to cross over to Cerezyme. Twenty-nine patients continued treatment for total duration of 24 months.
CT=computed tomography; MRI=magnetic resonance imaging.
Cerezyme showed similar reductions in visceral parameters at 6 months vs alglucerase1
Spleen volume
The mean baseline spleen volume was 2369 mL for Cerezyme patients and 2603 mL for alglucerase patients.
The absolute change from baseline was -902 mL for Cerezyme and -874 mL for alglucerase. Difference of absolute change was -28 mL (95% CI: -652, 596).
Liver volume
The mean baseline liver volume was 2521 mL for Cerezyme patients and 2788 mL for alglucerase patients.
The absolute change from baseline was -310 mL for Cerezyme and -307 mL for alglucerase. Difference of absolute change was -3 mL (95% CI: -246, 240).
aConfidence intervals were calculated using the t distribution (appropriate for small sample sizes) and the standard error of the difference in sample means (ie, the pooled estimate of the common standard deviation, computed as the weighted average of the standard deviations in the 2 treatment groups); there was no evidence that the assumption of equal variances between the groups was violated.
Cerezyme showed similar improvements in hematologic parameters at 6 months vs alglucerase1
Hemoglobin level
The mean baseline hemoglobin level was 10.7 g/dL for Cerezyme patients and 10.9 g/dL for alglucerase patients.
Platelet count
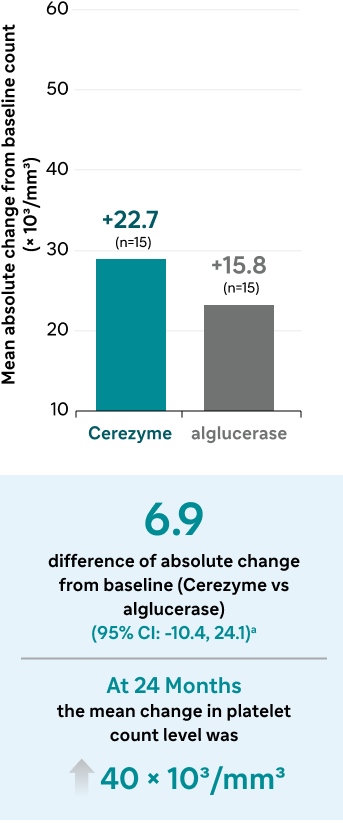
The mean baseline platelet count level was 68.5 × 103/mm3 for Cerezyme patients and 74.2 × 103/mm3 for alglucerase patients.
aConfidence intervals were calculated using the t distribution (appropriate for small sample sizes) and the standard error of the difference in sample means (ie, the pooled estimate of the common standard deviation, computed as the weighted average of the standard deviations in the 2 treatment groups); there was no evidence that the assumption of equal variances between the groups was violated.
Bone X-rays showed improvements in cortical thickness and lucencies in 7 of 11 Cerezyme-treated patients.
Indication
References: 1. Cerezyme (imiglucerase). Prescribing information. Genzyme Corporation, Cambridge, MA. 2. Grabowski GA, Barton NW, Pastores G, et al. Enzyme therapy in type 1 Gaucher disease: comparative efficacy of mannose-terminated glucocerebrosidase from natural and recombinant sources. Ann Intern Med. 1995;122(1):33-39.
