Jump to:
Recognize Gaucher disease and understand the consequences of delayed diagnosis and treatment.
Recognizing symptoms of Gaucher disease
Gaucher disease is an inherited, autosomal recessive lifelong condition marked by extreme diversity in genotype, phenotype, age of onset, and disease severity, as well as an unpredictable, progressive disease course.1,2 Signs, symptoms, and clinical course may differ even among individuals with the same genotype and within the same family.3 Patients may appear to be asymptomatic, yet harbor disease manifestations such as cytopenia, splenomegaly, or osteopenia.4,5 Symptoms should not be ignored, as this progressive condition may lead to further medical complications.4
Symptoms are diverse, unpredictable, and variable4-6
Onset may occur at any age.
Some patients may be asymptomatic while others may experience one or more symptoms.
The nature and severity of some symptoms may fluctuate as disease progresses.
Signs and symptoms
Gaucher disease is characterized by visceral, hematologic, skeletal, and neurologic symptoms. Patients do not always have all symptoms, and which symptoms they experience varies by disease subtype.2,7-9
Symptom categories by disease subtype2,7-9,a
|
Visceral |
Hematologic |
Skeletal |
Neurologic |
Cardiovascular
| |
| Type 1 |
X |
X |
X |
|
|
| Type 3 |
X |
X |
Xb |
X |
Xc |
| Type 2 |
X |
X |
|
X |
|
aPatients don’t always have all symptoms.
bIncluding kyphosis.10
cOnly in patients with GD3c subtype.11
Incidence of manifestations
Among patients with Gaucher disease:

90% present with splenomegaly6,12
60% to 80% present with hepatomegaly6,12
60% to 90% experience thrombocytopenia6,12
<10% experience neurologic symptoms12,13,a
aFor Gaucher patients in the US.13
All patients are at risk for skeletal complications
According to analyses of data from the International Collaborative Gaucher Group (ICGG) Gaucher Registry:

82% of patients have radiographic evidence of bone disease8
55% of patients have osteopenia14
16% of patients show evidence of osteonecrosis8

Up to 42% of children experience growth delay8
Monitor all patients diagnosed with Gaucher disease type 1 for the possible emergence of late onset neurologic symptoms; these patients may be reclassified to Gaucher disease type 3.11,15
Consequences of delayed diagnosis
Signs and symptoms of Gaucher disease can mimic more common childhood diseases, contributing to diagnostic delays that can exceed 7 years. Some patients receive misdiagnosis of conditions such as leukemia, multiple myeloma, or liver cancer.10
Gaucher disease types 1 and 3 can result in progressive visceral, hematologic, and skeletal manifestations if left untreated.15 Over time, these patients may experience:
- End-stage liver disease1
- Unnecessary splenectomy6
- Excessive bleeding6
- Blood transfusion dependence17
- Irreversible bone disease13
- Increased risk of bone fracture18
- Childhood growth delays19
- Emotional distress20
- Stress around major life decisions20
Early detection, intervention, and management of Gaucher disease are key to helping prevent progressive visceral and skeletal damage.1,8,10
Suspect Gaucher disease? Test to know
- β-glucosidase enzyme assay is the standard, recommended method for establishing a confirmatory diagnosis of Gaucher disease, which is demonstrated by deficiency of β-glucosidase activity5
- Molecular testing (DNA testing) can be used to establish a diagnosis of Gaucher disease, as well as carrier status5,21
- A bone marrow biopsy is typically not necessary or recommended to diagnose Gaucher disease because although bone marrow biopsy specimens can rule out hematologic malignancies, they are not reliable for establishing the presence of Gaucher cells4,5
This diagnostic algorithm may help you know when to test for Gaucher disease5
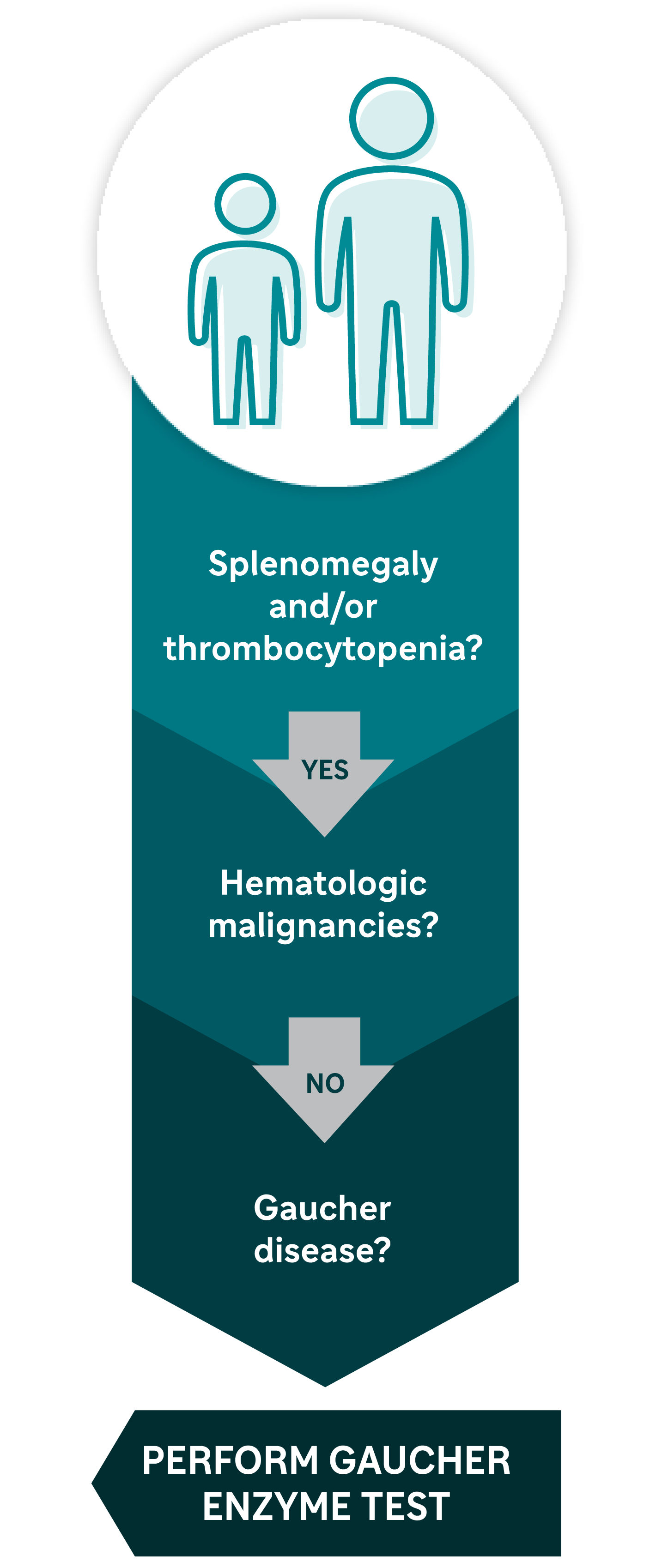
Don’t miss a Gaucher disease diagnosis. Test to know. Blood-based enzyme assay (β-glucosidase) is the gold standard for definitive diagnosis of Gaucher.5,a
aThe above algorithm is for the general population. Gaucher disease type 1 is more prevalent in individuals of Ashkenazi Jewish descent and should be investigated before hematologic malignancies.11
ERT=enzyme replacement therapy.
Indication
References: 1. Carubbi F, Cappellini MD, Fargion S, Fracanzani AL, Nascimbeni F. Liver involvement in Gaucher disease: a practical review for the hepatologist and the gastroenterologist. Dig Liver Dis. 2020;52(4):368-373. 2. Schiffmann R, Sevigny J, Rolfs A, et al. The definition of neuronopathic Gaucher disease. J Inherit Metab Dis. 2020;43(5):1056-1059. 3. Lachmann RH, Grant IR, Halsall D, Cox TM. Twin pairs showing discordance of phenotype in adult Gaucher’s disease. QJM. 2004;97(4):199-204. 4. Mistry PK, Sadan S, Yang R, Yee J, Yang M. Consequences of diagnostic delays in type 1 Gaucher disease: the need for greater awareness among hematologists-oncologists and an opportunity for early diagnosis and intervention. Am J Hematol. 2007;82(8):697-701. 5. Mistry PK, Cappellini MD, Lukina E, et al. A reappraisal of Gaucher disease-diagnosis and disease management algorithms. Am J Hematol. 2011;86(1):110-115. 6. Stirnemann J, Belmatoug N, Camou F, et al. A review of Gaucher disease pathophysiology, clinical presentation and treatments. Int J Mol Sci. 2017;18(2):441. 7. Deegan PB, Cox TM. Imiglucerase in the treatment of Gaucher disease: a history and perspective. Drug Des Devel Ther. 2012;6:81-106. 8. Goker-Alpan O. Therapeutic approaches to bone pathology in Gaucher disease: past, present and future. Mol Genet Metab. 2011;104(4):438-447. 9. El-Beshlawy A, Abdel-Azim K, Abdel-Salam A, et al. Egyptian Gaucher disease type 3 patients: a large cohort study spanning two decades. J Rare Dis. 2023;2(7). doi:10.1007/s44162-023-00011-0 10. Weinreb NJ, Goker-Alpan O, Kishnani PS, et al. The diagnosis and management of Gaucher disease in pediatric patients: where do we go from here? Mol Genet Metab. 2022;136(1):4-21. 11. Zhong W, Li D, Fei Y, Hong P. A review of type 3 Gaucher disease: unique neurological manifestations and advances in treatment. Acta Neurol Belg. 2024;124(4):1213-1223. 12. Charrow J, Andersson HC, Kaplan P, et al. The Gaucher registry: demographics and disease characteristics of 1698 patients with Gaucher disease. Arch Intern Med. 2000;160(18):2835-2843. 13. El-Beshlawy A, Tylki-Szymańska A, Vellodi A, et al. Long-term hematological, visceral, and growth outcomes in children with Gaucher disease type 3 treated with imiglucerase in the International Collaborative Gaucher Group Gaucher Registry. Mol Genet Metab. 2017;120(1-2):47-56. 14. Hughes D, Mikosch P, Belmatoug N, et al. Gaucher disease in bone: from pathophysiology to practice. J Bone Miner Res. 2019;34(6):996-1013. 15. Giraldo P, Alfonso P, Irún P, et al. Mapping the genetic and clinical characteristics of Gaucher disease in the Iberian Peninsula. Orphanet J Rare Dis. 2012;7:17. doi:10.1186/1750-1172-7-17 16. Lu WL, Chien YH, Tsai FJ, et al. Changing clinical manifestations of Gaucher disease in Taiwan. Orphanet J Rare Dis. 2023;18(1):293. 17. Gary SE, Ryan E, Steward AM, Sidranksy E. Recent advances in the diagnosis and management of Gaucher disease. Expert Rev Endocrinol Metab. 2018;13(2):107-118. 18. Deegan P, Khan A, Camelo JS Jr, Batista JL, Weinreb N. The International Collaborative Gaucher Group GRAF (Gaucher Risk Assessment for Fracture) score: a composite risk score for assessing adult fracture risk in imiglucerase-treated Gaucher disease type 1 patients. Orphanet J Rare Dis. 2021;16(1):92. 19. Kauli R, Zaizov R, Lazar L, et al. Delayed growth and puberty in patients with Gaucher disease type 1: natural history and effect of splenectomy and/or enzyme replacement therapy. Isr Med Assoc J. 2000;2(2):158-163. 20. Packman W, Crosbie TW, Behnken M, Eudy K, Packman S. Living with Gaucher disease: emotional health, psychosocial needs and concerns of individuals with Gaucher disease. Am J Med Genet A. 2010;152A(8):2002-2010. 21. Grabowski GA, Petsko GA, Kolodny Eh. Gaucher disease. In: Valle DL, Antonarakis S, Bellabio A, Beaudet AL, Mitchell GA. eds. The Online Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill Education; 2019. Accessed March 11, 2024. https://ommbid.mhmedical.com/content.aspx?bookid=2709§ionid=225546056
