CV Evaluation from the ORIGIN Trial over 6.2 Years (N=12,537)
Limitations:
- Metformin was used by 47% of the insulin-glargine group and 60% of the standard-care group which may have been cardioprotective.

- The trial involved people who were not normally prescribed insulin and in whom insulin glargine was used to achieve fasting plasma glucose levels much lower than those typically achieved with insulin therapy. However, the effect in participants with and those without diabetes was similar.
- The trial was designed to test the effect of using titrated basal insulin to control glucose levels vs standard-care with guideline-suggested degrees of glycemic control; the trial was specifically not designed to test more intense vs less intense glucose lowering.
No statistically significant differences between Lantus and standard care in the co-primary endpoints
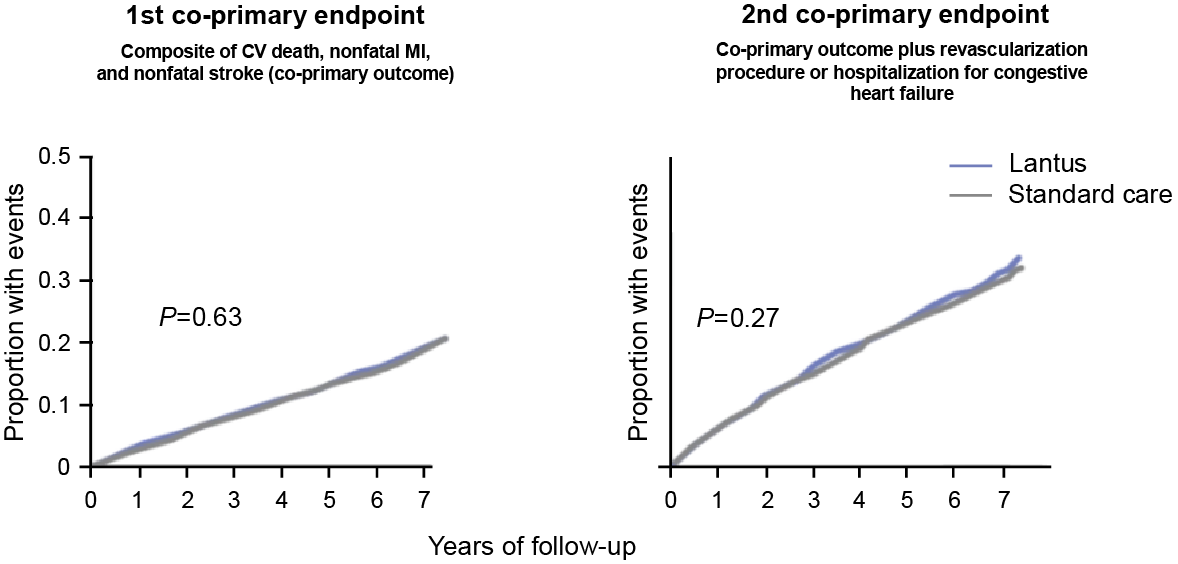
- Similar results were observed between Lantus and standard care for all cause mortality
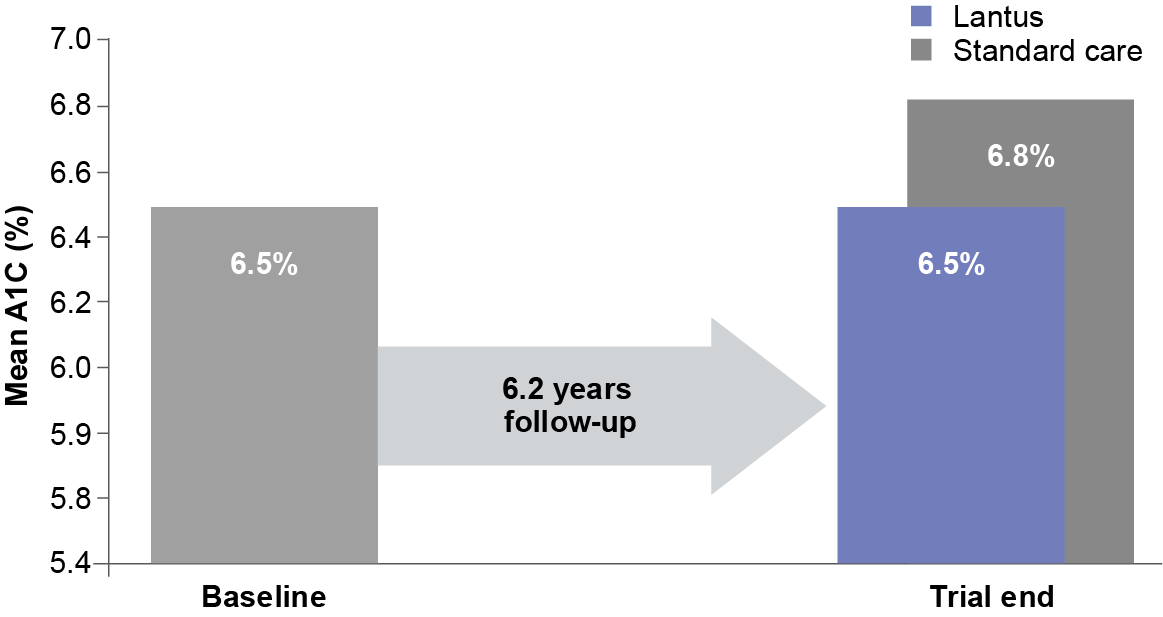
Mean A1C over 6.2 years (median)
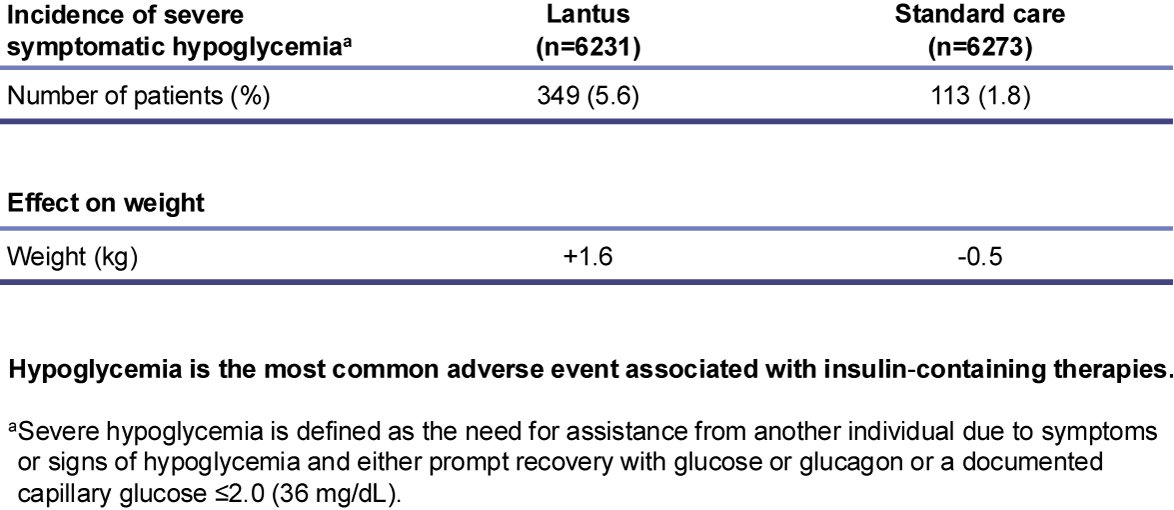
Safety results
.png)
Study Design
A randomized study of patients with IFG (Impaired fasting glucose) and/or IGT (Impaired glucose tolerance) or early type 2 diabetes mellitus and established CV disease or CV risk at baseline. Patients received either Lantus (n=6264) titrated to a target FPG of ≤95 mg/dL or standard care (n=6273). Standard care patients remained on their original treatment, if applicable, at randomization. Additional agents could be added as needed at the discretion of the investigator. The co-primary outcomes were nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes and these events plus revascularization or hospitalization for heart failure. Microvascular outcomes, incident diabetes, hypoglycemia, weight, and cancers were also compared between groups. Median follow-up: 6.2 years.
Important Safety Information
References:
1. Lantus Prescribing Information.
2. Origin Trial Investigators, Gerstein H, Yusuf S, Riddle MC, Ryden L, Bosch J. Am Heart J. 2008;155(1):26-32.e326.
3. ORIGIN Trial Investigators, Gerstein HC, Bosch J, et al. N Engl J Med. 2012;367(4):319-328.