Lantus was evaluated for 52 weeks in adults with type 2 diabetes poorly controlled with OADs
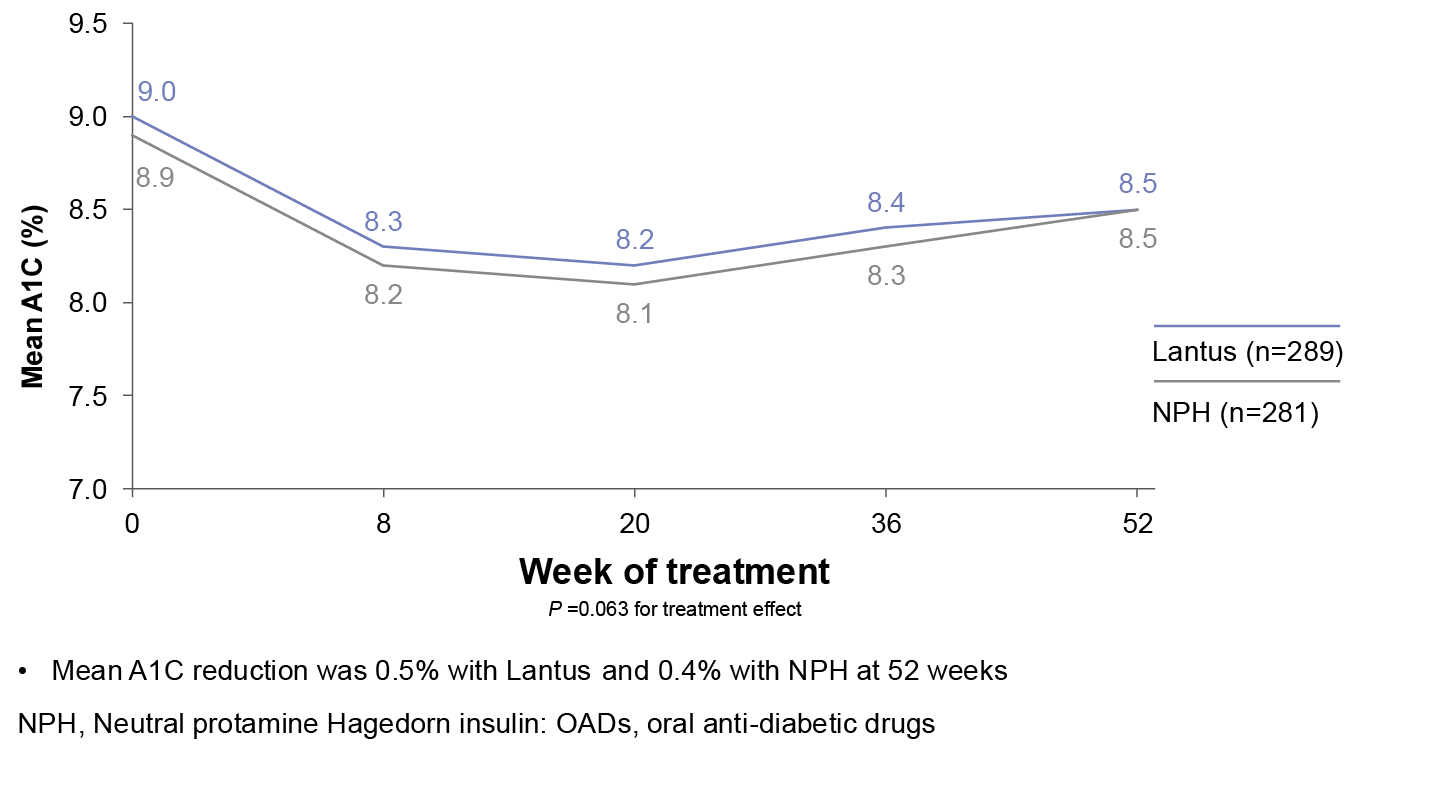
Mean A1C Levels
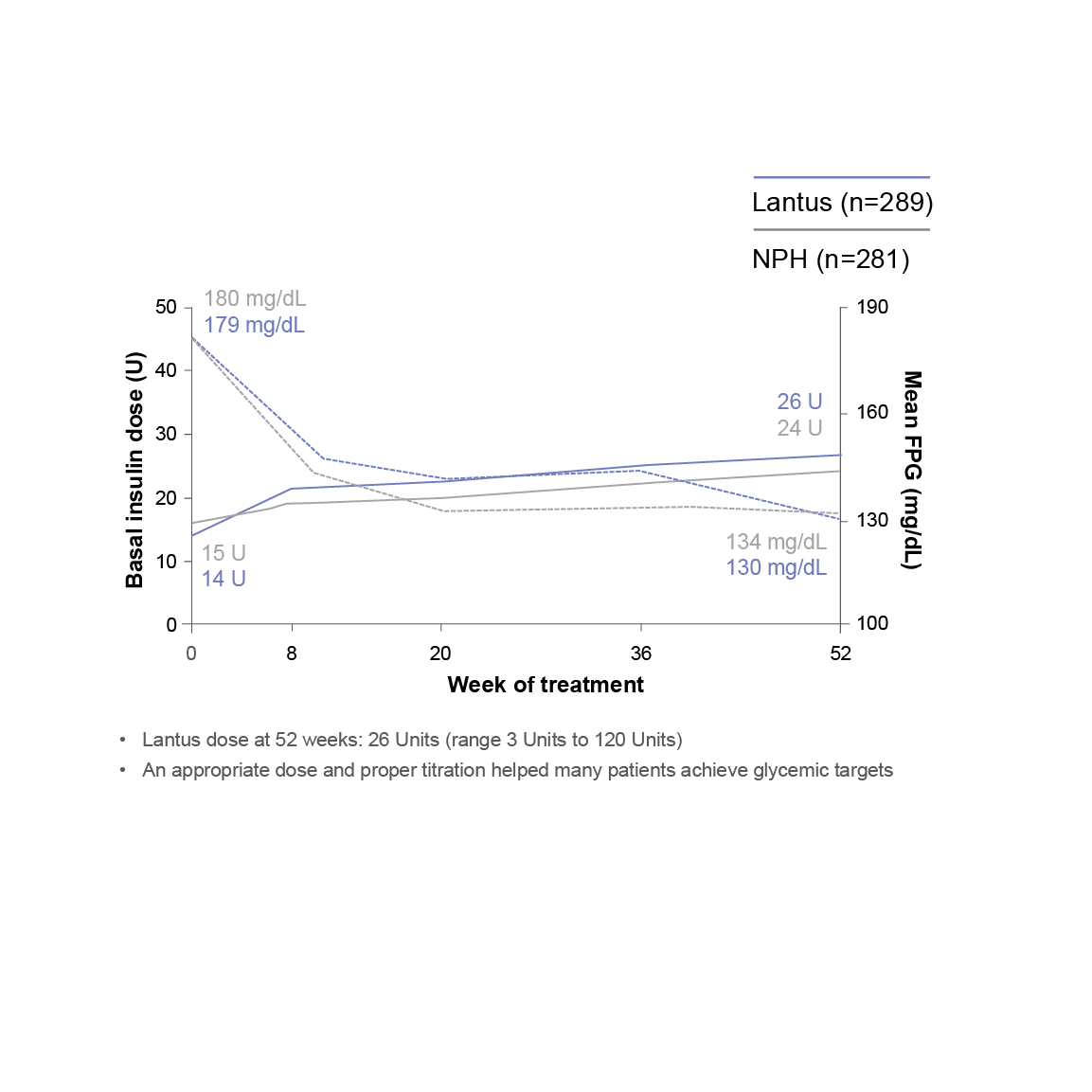
Insulin dose and reductions in FPG (Fasting plasma glucose) during a 52-week study
.png)
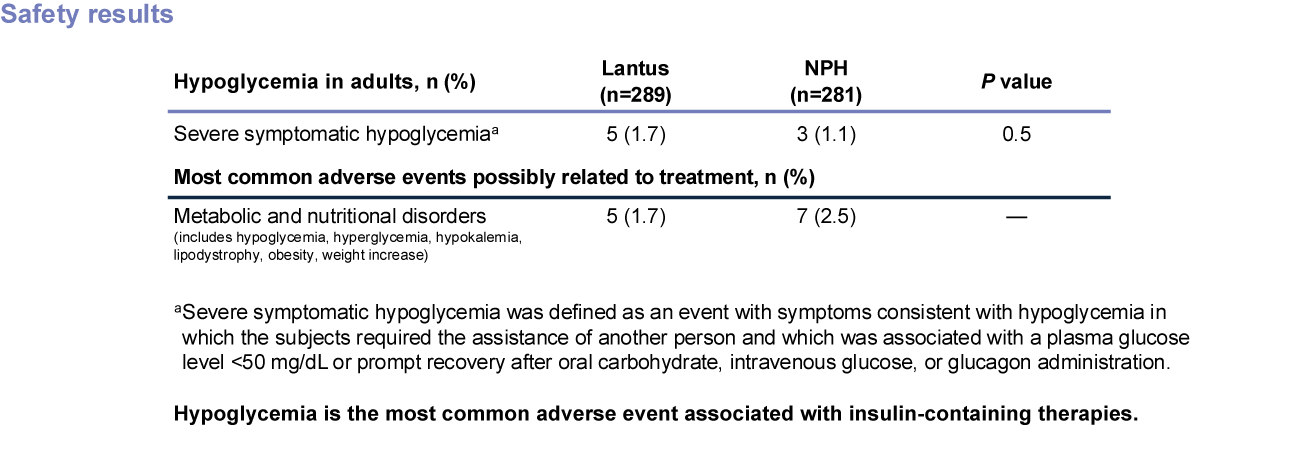
Hypoglycemia is the most common adverse event associated with insulin-containing therapies.
Study Design
A 52-week randomized study (N=570) designed to compare efficacy and safety of Lantus plus OADs vs NPH plus OADs in patients with type 2 diabetes poorly controlled with OADs. Patients were randomized to either Lantus (n=289) or NPH (n=281) at bedtime to reach a target FPG of <120 mg/dL. OADs were continued. Initial insulin dose and titration schedule were left to the discretion of the individual investigators. Primary endpoint was change in A1C.
IN A TREAT-TO-TARGET PEDIATRIC TRIAL WITH NPH1,3
In a pediatric clinical study, children and adolescents with T1DM had a higher incidence of severe symptomatic hypoglycemia in the 2 treatment groups (Lantus or NPH) compared to adult trials with type 1 diabetes.
- Baseline A1C was 8.48% and 8.81% for the Lantus and NPH groups, respectively
- The relative change in A1C at 28 weeks was +0.28% for the Lantus group (n=174) and +0.27% for the NPH group (n=175)
Once-daily pediatric dosing
In the same pediatric trial
- 35% of patients on NPH needed to inject twice as often as patients on once-daily Lantus in order to maintain comparable A1C levels
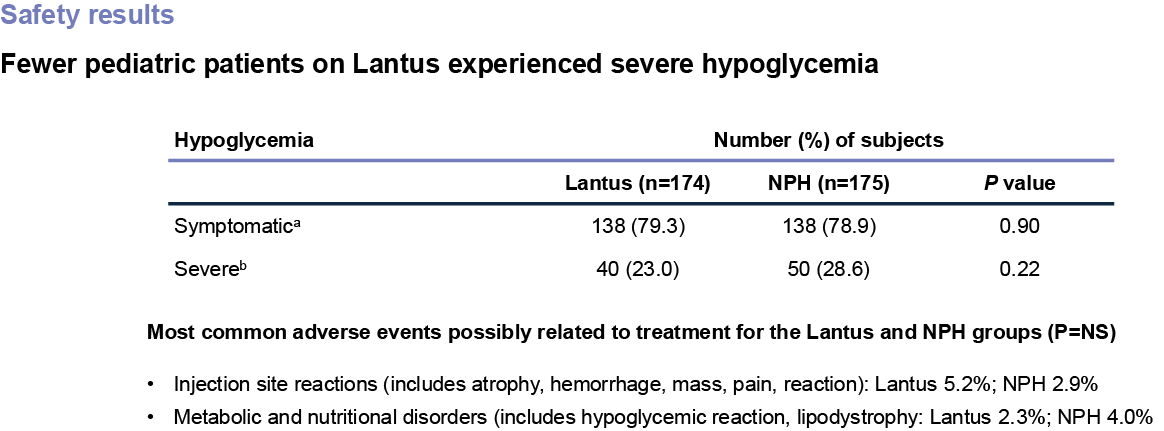
Hypoglycemia is the most common adverse event associated with insulin-containing therapies.
Study Design
A 28-week, randomized, open-label, multicenter study of 349 patients with type 1 diabetes (aged 6-15) who received once-daily Lantus (n=174) or once- or twice-daily NPH (n=175) in combination with regular human insulin as the mealtime insulin. The primary efficacy measure was mean change in A1C from baseline.
aSymptomatic hypoglycemia was defined as any event with clinical symptoms that could be confirmed by BG level <50 mg/dL.
bSevere hypoglycemia was defined as an event with symptoms consistent with hypoglycemia in which the subjects required the assistance of another person and which was associated with a BG level <50 mg/dL or prompt recovery after oral carbohydrate, intravenous glucose, or glucagon administration. This definition is consistent with that used in the Diabetes Control and Complications Trial.
Important Safety Information
References:
1. Lantus Prescribing Information.
2. Yki-Järvinen H, Dressler A, Ziemen M, HOE 901/300s Study Group. Diabetes Care. 2000;23(8):1130-1136.
3. Schober E, Schoenle E, Van Dyk J, Wernicke-Panten K. Pediatric Study Group of Insulin Glargine. J Pediatr Endocrinol Metab. 2002;15(4):369-376.