Durable and rapid platelet response with WAYRILZ vs placebo control1
PRIMARY ENDPOINT:
Significant Durable Platelet Response by Week 251
A platelet count ≥50x109/L for ≥5 of at least 8 non-missing weekly measurements during the last 12 weeks of the DB period, including ≥2 such responses in the last 6 weeks without rescue therapy
Faster and longer-lasting response vs placebo control was shown in all patients treated with WAYRILZ1,2
Time to first platelet response1,2*
36 DAYS All WAYRILZ Arm (n=133)
(vs placebo control, p<0.0001)
NOT REACHED Placebo Control (n=69)
15 DAYS WAYRILZ Responders† (n=85)
Number of weeks with platelet response1,2‡
7 WEEKS All WAYRILZ Arm (n=133)
(vs placebo control, p<0.0001)
<1 WEEK Placebo Control (n=69)
11 WEEKS WAYRILZ Responders† (n=85)
*Platelet count ≥50x109/L or between 30x109/L and <50x109/L and at least doubled from baseline in the absence of rescue therapy.1
†Responders had ≥1 platelet response of at least 50x109/L or ≥30x109/L and <50x109/L and at least doubled from baseline by Week 13 without rescue therapy.2
‡Platelet count ≥50x109/L or between 30x109/L and <50x109/L and doubled from baseline. Platelet counts assessed within 4 weeks of rescue medication intake are considered as no response; missing weekly platelet counts due to any reasons are considered as no response.1,2
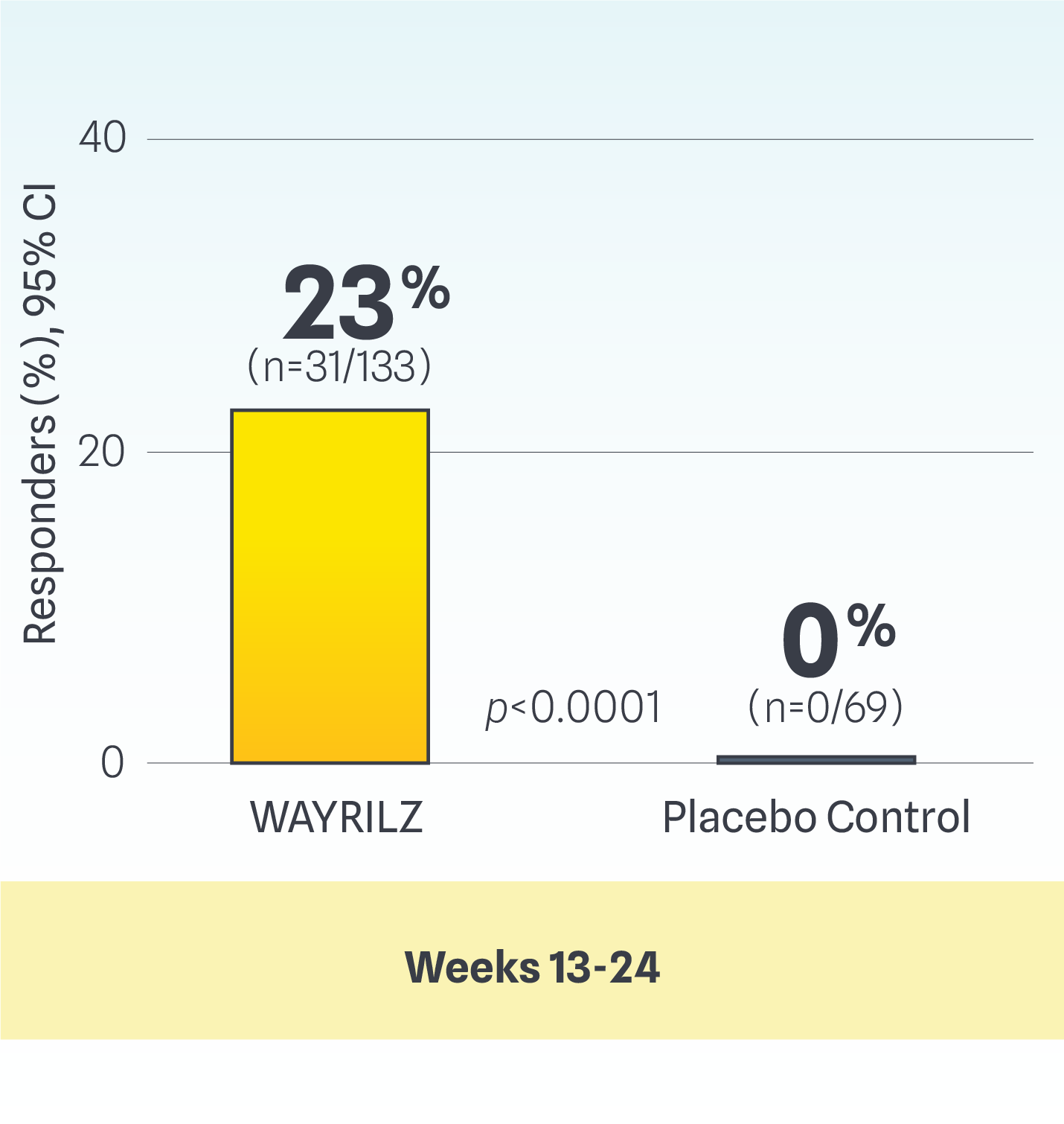
Post-hoc analysis: modified durable response
Modified durable response: Defined using the International Working Group (IWG)* standard for platelet response as part of the criteria: a platelet count of ≥30×109/L and at least doubled from baseline, in the absence of bleeding, for ≥50% of assessments during the last 12 weeks of the DB period or the last 16 weeks of the OL period, provided that ≥6 or ≥8 non-missing platelet counts were available in the DB and OL periods, respectively.3
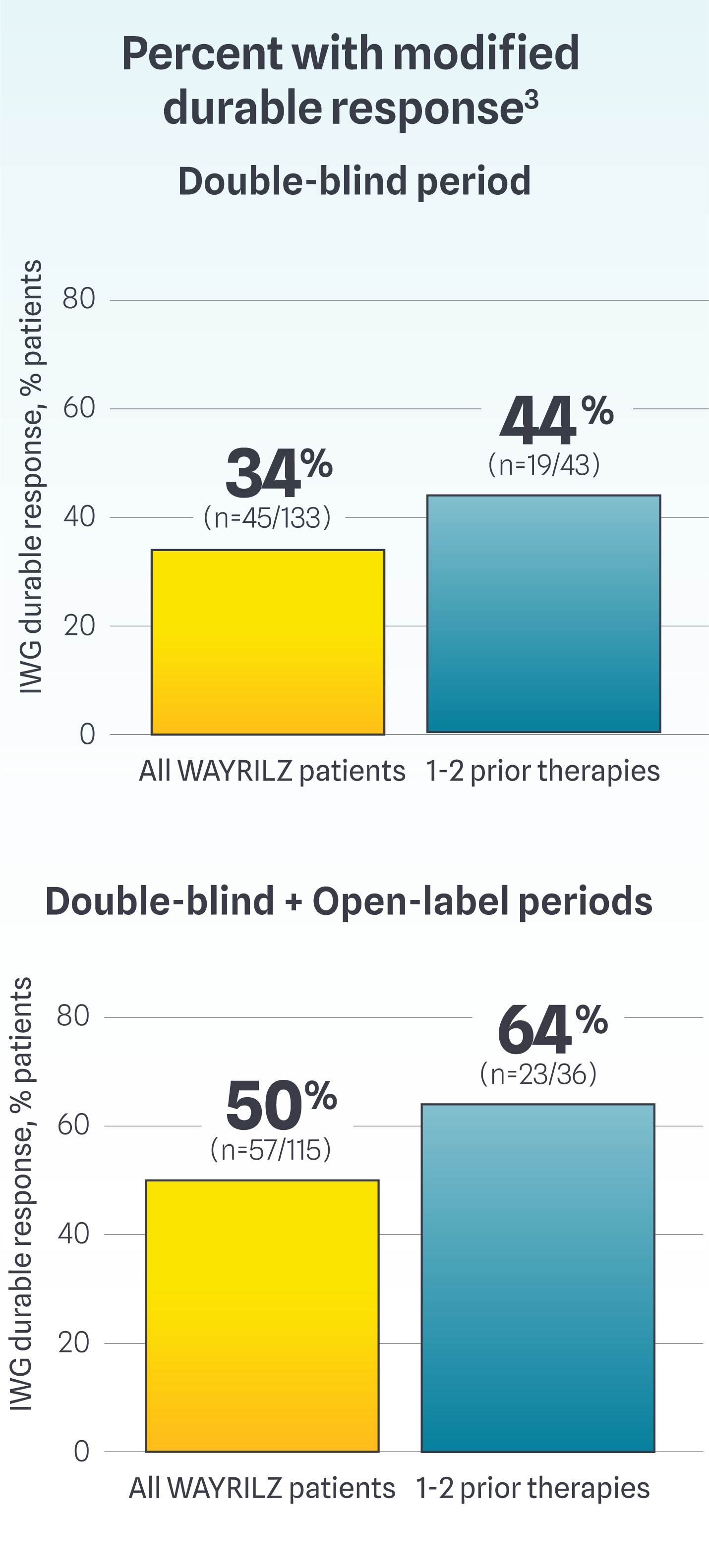
Study design: Data are from a post-hoc analysis of patients with persistent or chronic ITP who were enrolled in the LUNA-3 clinical study. Analyses were conducted by applying a modified durable platelet response criteria while preserving the randomization from the LUNA-3 study. Modified durable response using the IWG standard for platelet response as part of the criteria.4
Study limitations: This is a post-hoc analysis that was not designed or powered to establish statistical significance. Results are descriptive only and definitive conclusions cannot be made.
*To address the need for standardization of response criteria with the introduction of novel targeted therapies, the IWG has established standardized terminology, definitions, and outcome criteria for ITP. The IWG criteria define platelet response thresholds that are clinically meaningful and safe, aiming to guide treatment decisions and bleeding risk.5
Median Platelet Counts Throughout the Double-blind and Open-label Periods
Durable responders achieved median counts exceeding 30x109/L, 50x109/L, and 100x109/L at Weeks 2, 3, and 14, respectively, and maintained >100x109/L thereafter. Nondurable responders achieved median counts >30x109/L from Weeks 14 to 25. In the OL period, some WAYRILZ DB nondurable responders were able to achieve median platelet counts above ~60x109/L.2,6
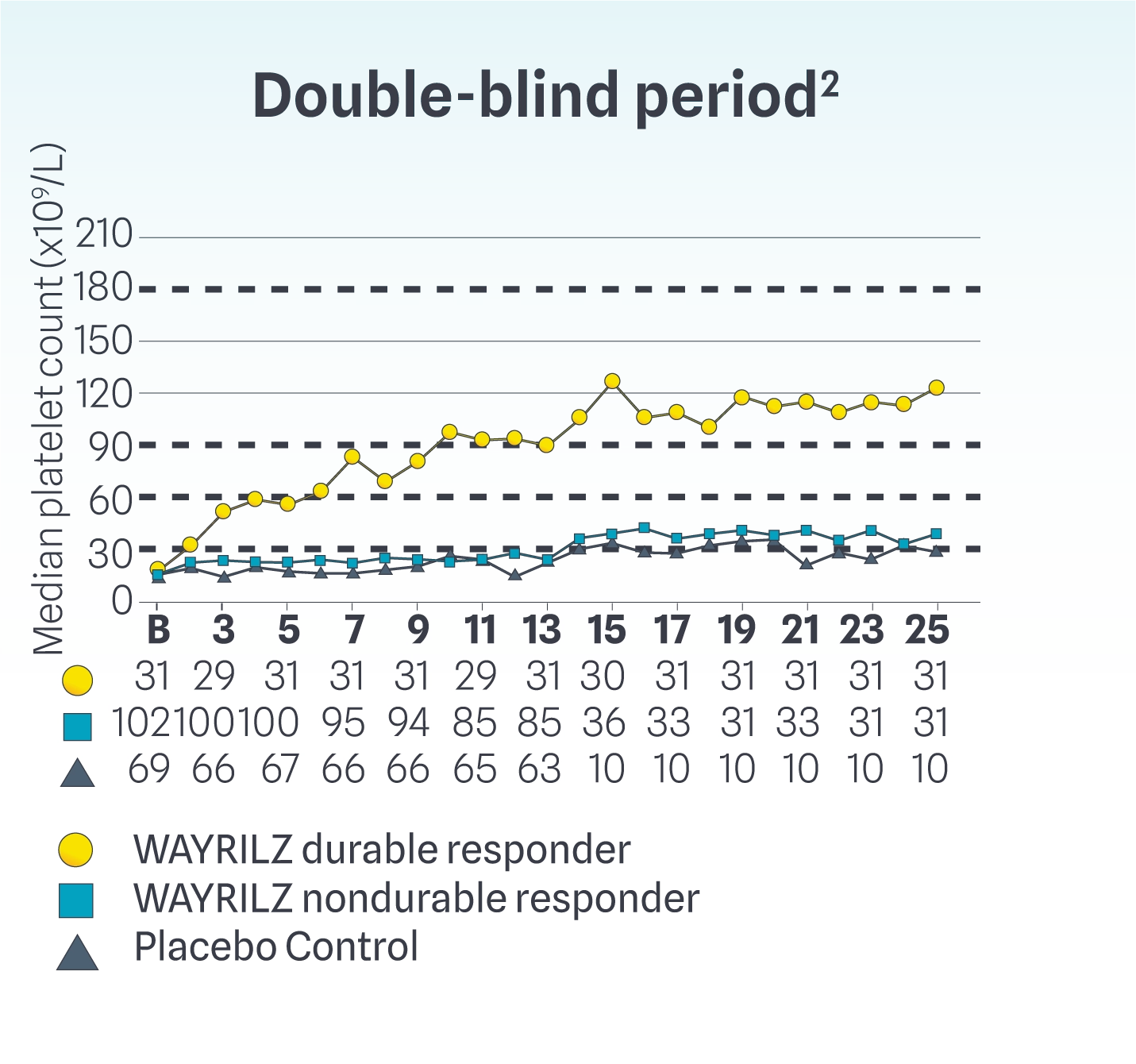
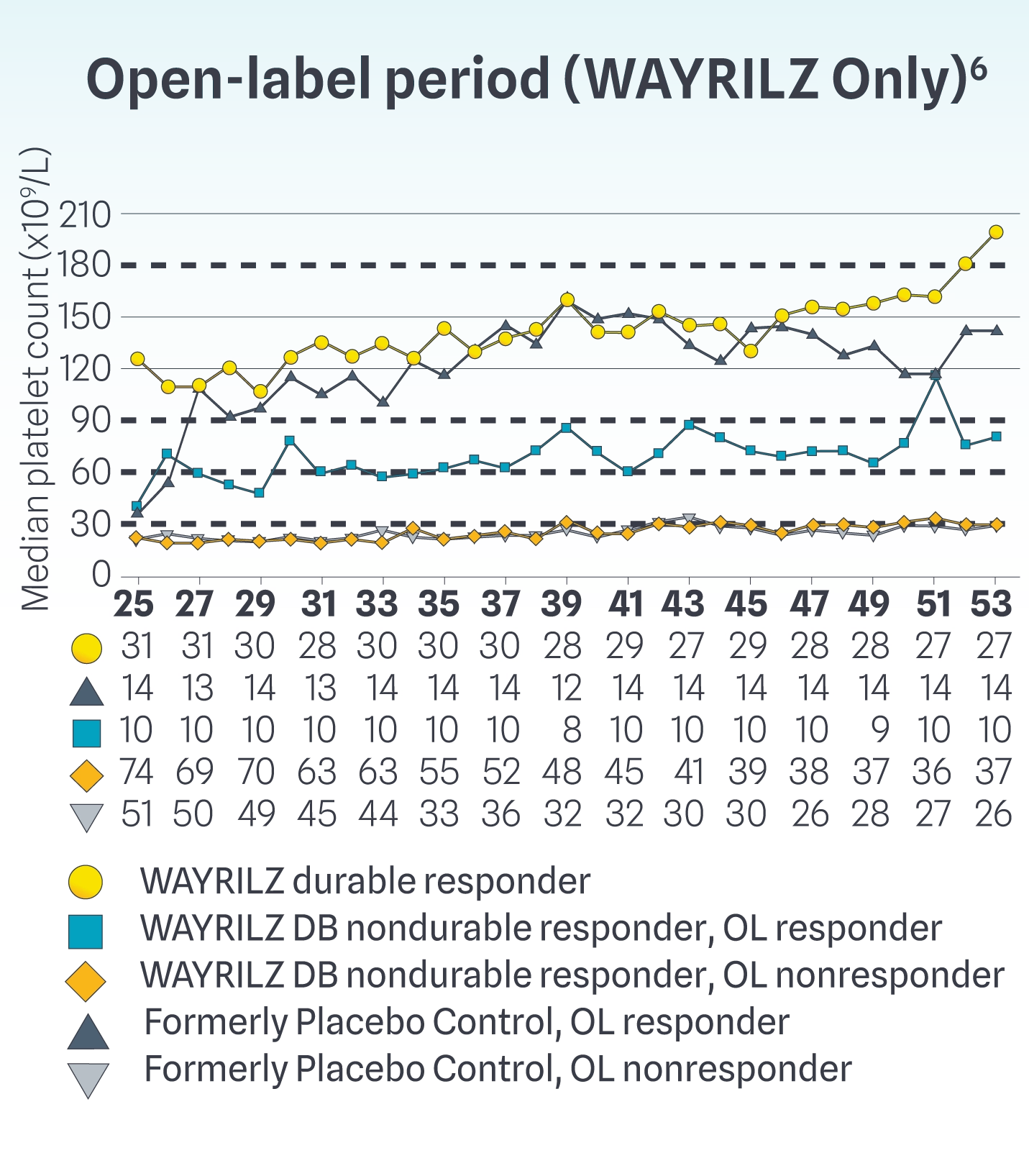
Results are descriptive only. Definitive conclusions cannot be made. Limitations associated with open-label study design include lack of comparator arm, decreasing sample size, and potential continued involvement of responders and attrition of nonresponders.
WAYRILZ Durable Responders: Patients achieving a platelet count of ≥50x109/L for ≥5 of at least 8 non-missing weekly measurements during the last 12 weeks of the DB period, including ≥2 such responses in the last 6 weeks without rescue therapy.1
WAYRILZ Nondurable Responders: Patients not achieving a platelet count of ≥50x109/L for ≥5 of at least 8 non-missing weekly measurements during the last 12 weeks of the DB period, including ≥2 such responses in the last 6 weeks without rescue therapy.1
Rescue Therapy Use and Bleeding Assessment Scores
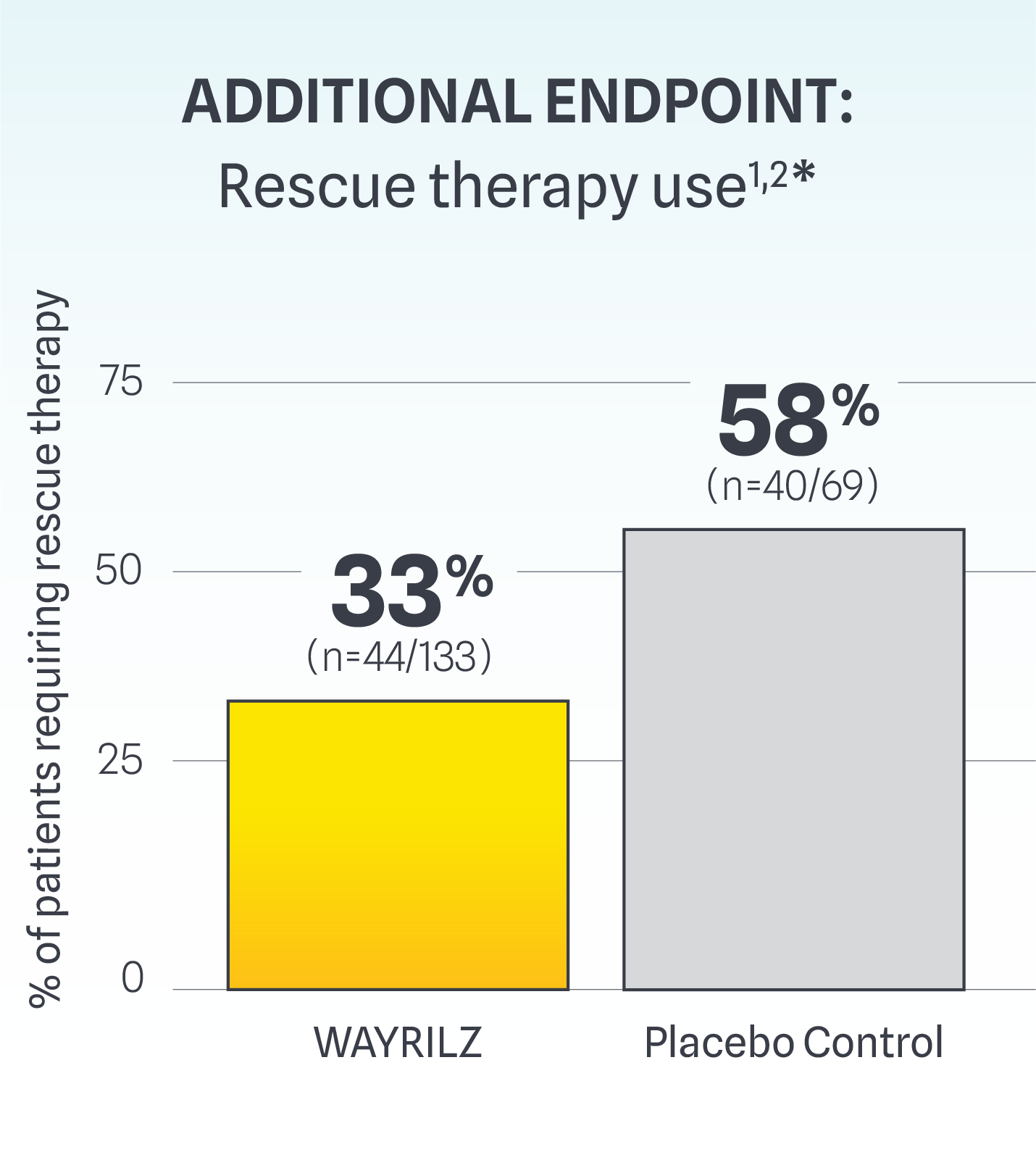
Fewer patients taking WAYRILZ required rescue therapy use throughout the double-blind period.
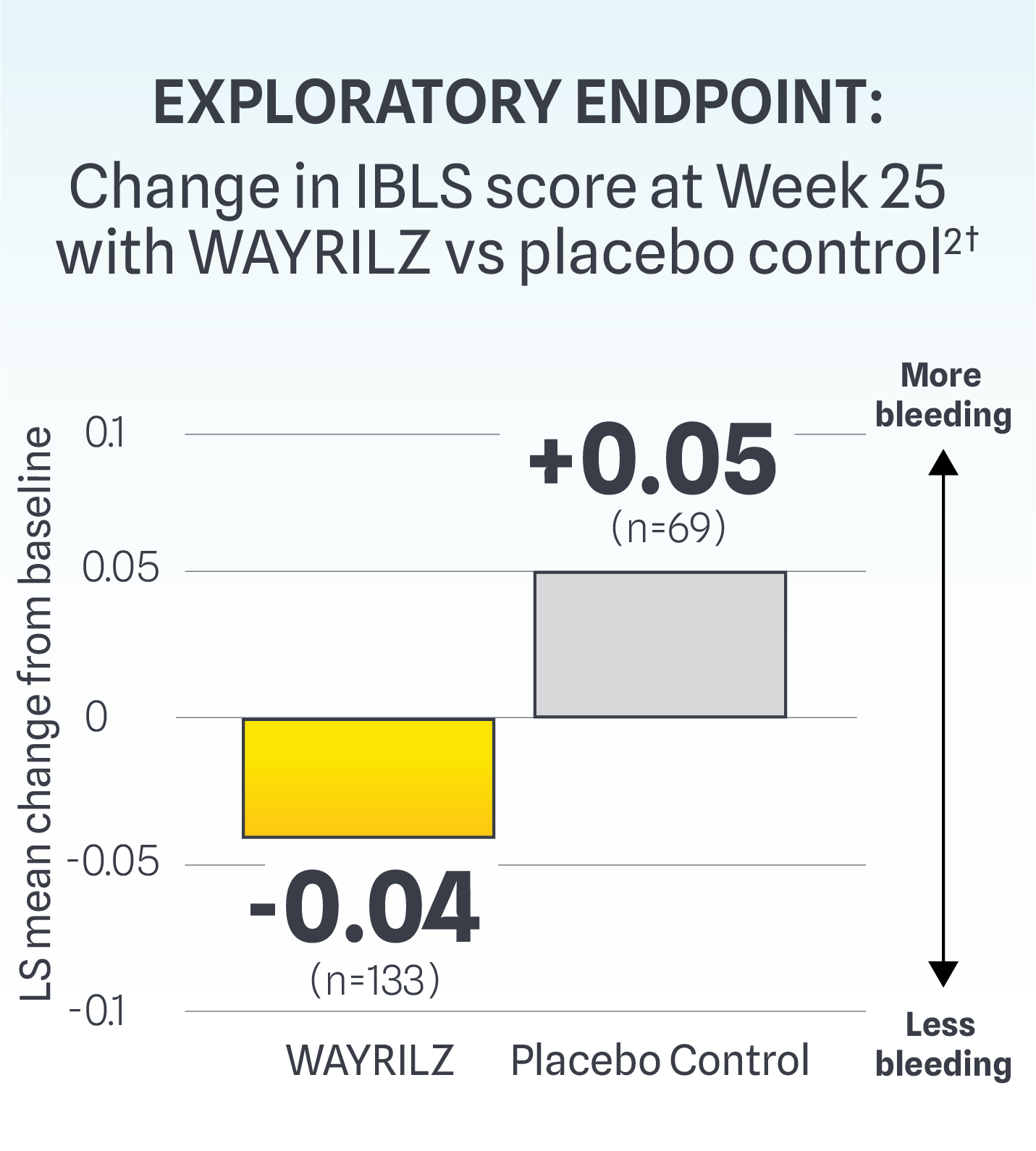
LS mean difference: -0.09
This exploratory analysis was not designed or powered to detect differences between treatment groups.
*Rescue therapy use was assessed regardless of the criteria for durable response.1,2
†As measured by the ITP Bleeding Scale (IBLS), which was designed to assess and quantify bleeding symptoms in patients with ITP. It includes 11 site-specific grades to evaluate bleeding severity across different body areas.2
Changes in HRQoL Domains As Measured By The ITP-PAQ2
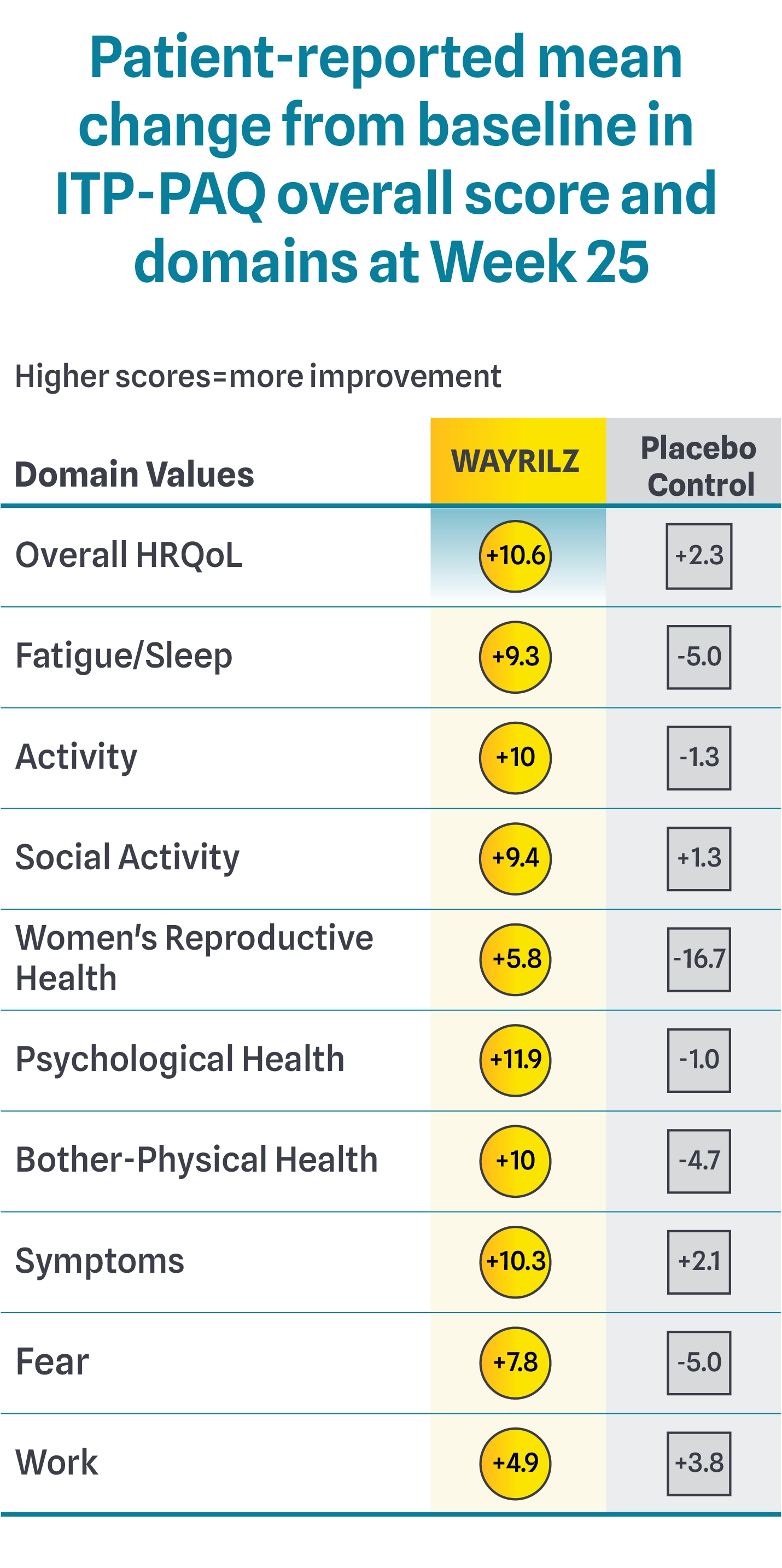
The Immune Thrombocytopenia Patient Assessment Questionnaire (ITP-PAQ) assesses domains including overall HRQoL, fatigue/sleep, activity, social activity, women's reproductive health, psychological health, bother-physical health, symptoms, fear, and work. The results of these analyses are descriptive only and were not powered for statistical significance. The patient-reported nature of the data may impact the reliability of findings. Definitive conclusions cannot be made.
Durable and rapid platelet response with WAYRILZ vs placebo control1
PRIMARY ENDPOINT:
Significant Durable Platelet Response by Week 251
A platelet count ≥50x109/L for ≥5 of at least 8 non-missing weekly measurements during the last 12 weeks of the DB period, including ≥2 such responses in the last 6 weeks without rescue therapy

Faster and longer-lasting response vs placebo control was shown in all patients treated with WAYRILZ1,2
Time to first platelet response1,2*
36 DAYS All WAYRILZ Arm (n=133)
(vs placebo control, p<0.0001)
NOT REACHED Placebo Control (n=69)
15 DAYS WAYRILZ Responders† (n=85)
Number of weeks with platelet response1,2‡
7 WEEKS All WAYRILZ Arm (n=133)
(vs placebo control, p<0.0001)
<1 WEEK Placebo Control (n=69)
11 WEEKS WAYRILZ Responders† (n=85)
*Platelet count ≥50x109/L or between 30x109/L and <50x109/L and at least doubled from baseline in the absence of rescue therapy.1
†Responders had ≥1 platelet response of at least 50x109/L or ≥30x109/L and <50x109/L and at least doubled from baseline by Week 13 without rescue therapy.2
‡Platelet count ≥50x109/L or between 30x109/L and <50x109/L and doubled from baseline. Platelet counts assessed within 4 weeks of rescue medication intake are considered as no response; missing weekly platelet counts due to any reasons are considered as no response.1,2
Post-hoc analysis: modified durable response
Modified durable response: Defined using the International Working Group (IWG)* standard for platelet response as part of the criteria: a platelet count of ≥30×109/L and at least doubled from baseline, in the absence of bleeding, for ≥50% of assessments during the last 12 weeks of the DB period or the last 16 weeks of the OL period, provided that ≥6 or ≥8 non-missing platelet counts were available in the DB and OL periods, respectively.3

Study design: Data are from a post-hoc analysis of patients with persistent or chronic ITP who were enrolled in the LUNA-3 clinical study. Analyses were conducted by applying a modified durable platelet response criteria while preserving the randomization from the LUNA-3 study. Modified durable response using the IWG standard for platelet response as part of the criteria.4
Study limitations: This is a post-hoc analysis that was not designed or powered to establish statistical significance. Results are descriptive only and definitive conclusions cannot be made.
*To address the need for standardization of response criteria with the introduction of novel targeted therapies, the IWG has established standardized terminology, definitions, and outcome criteria for ITP. The IWG criteria define platelet response thresholds that are clinically meaningful and safe, aiming to guide treatment decisions and bleeding risk.5
Median Platelet Counts Throughout the Double-blind and Open-label Periods
Durable responders achieved median counts exceeding 30x109/L, 50x109/L, and 100x109/L at Weeks 2, 3, and 14, respectively, and maintained >100x109/L thereafter. Nondurable responders achieved median counts >30x109/L from Weeks 14 to 25. In the OL period, some WAYRILZ DB nondurable responders were able to achieve median platelet counts above ~60x109/L.2,6


Results are descriptive only. Definitive conclusions cannot be made. Limitations associated with open-label study design include lack of comparator arm, decreasing sample size, and potential continued involvement of responders and attrition of nonresponders.
WAYRILZ Durable Responders: Patients achieving a platelet count of ≥50x109/L for ≥5 of at least 8 non-missing weekly measurements during the last 12 weeks of the DB period, including ≥2 such responses in the last 6 weeks without rescue therapy.1
WAYRILZ Nondurable Responders: Patients not achieving a platelet count of ≥50x109/L for ≥5 of at least 8 non-missing weekly measurements during the last 12 weeks of the DB period, including ≥2 such responses in the last 6 weeks without rescue therapy.1
Rescue Therapy Use and Bleeding Assessment Scores

Fewer patients taking WAYRILZ required rescue therapy use throughout the double-blind period.

LS mean difference: -0.09
This exploratory analysis was not designed or powered to detect differences between treatment groups.
*Rescue therapy use was assessed regardless of the criteria for durable response.1,2
†As measured by the ITP Bleeding Scale (IBLS), which was designed to assess and quantify bleeding symptoms in patients with ITP. It includes 11 site-specific grades to evaluate bleeding severity across different body areas.2
Changes in HRQoL Domains As Measured By The ITP-PAQ2

The Immune Thrombocytopenia Patient Assessment Questionnaire (ITP-PAQ) assesses domains including overall HRQoL, fatigue/sleep, activity, social activity, women's reproductive health, psychological health, bother-physical health, symptoms, fear, and work. The results of these analyses are descriptive only and were not powered for statistical significance. The patient-reported nature of the data may impact the reliability of findings. Definitive conclusions cannot be made.
CI, confidence interval; DB, double-blind; HRQoL, health-related quality of life; ITP, immune thrombocytopenia; LS, least squares; MOA, mechanism of action; OL, open-label.
INDICATION
References: 1. WAYRILZ. Prescribing information. Sanofi, Inc. 2. Kuter DJ, Ghanima W, Cooper N, et al; LUNA3 Trial Group. Safety and efficacy of rilzabrutinib vs placebo in adults with immune thrombocytopenia: the phase 3 LUNA3 study. Blood. 2025;145(24):2914-2926. 3. Data on File. Sanofi; 2025. 4. Ghanima W, Miyakawa Y, Cooper N, et al. Platelet responses per IWG criteria for LUNA3 rilzabrutinib vs placebo in primary ITP patients. Abstract presented at: 33rd Congress of the International Society on Thrombosis and Haemostasis (ISTH); June 21-25, 2025; Washington, DC. 5. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393. 6. Kuter DJ, Ghanima W, Cooper N, et al. LUNA3 open-label period: first efficacy/safety with rilzabrutinib in previously treated ITP adults. Poster presented at: 33rd Congress of the International Society on Thrombosis and Haemostasis (ISTH); June 21-25, 2025; Washington, DC.