WAYRILZ: LUNA-3 study design1-3
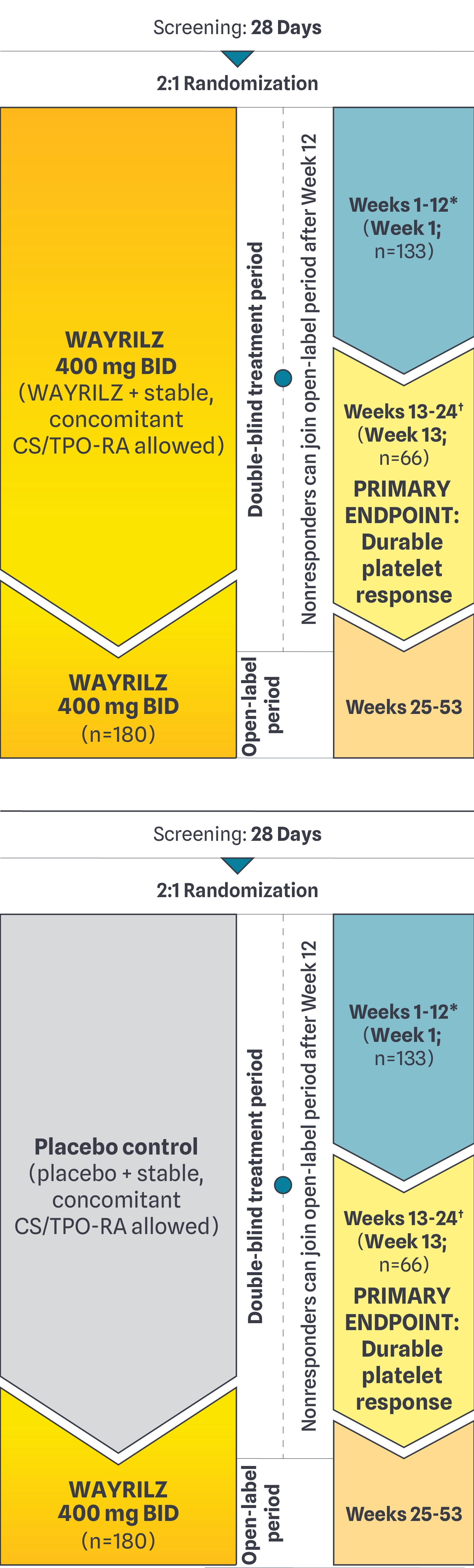
Response Criteria
To qualify for continuation in the study through the end of the
DB period, patients had to have ≥1 platelet response of at least
50x109/L or ≥30x109/L and <50x109/L and at least doubled
from baseline by Week 13 without rescue therapy.1,2*
PRIMARY ENDPOINT: Durable platelet response
A platelet count ≥50x109/L for ≥5 of at least 8 non-missing weekly measurements during the last 12 weeks of the DB period, including ≥2 such responses in the last 6 weeks without rescue therapy.1*

At Week 13, 64% of patients receiving WAYRILZ were responders (n=85) compared to 32% of patients in the placebo control group (n=22).1,2
*Rescue therapy= intravenous immunoglobulin (IVlg), high-dose CS, platelet infusion, or anti-D immunoglobulin infusion (anti-D).
WAYRILZ was studied in a wide range of patients1,2*
LUNA-3: A phase 3, randomized, double-blind, placebo-controlled, parallel-group, multicenter study to evaluate the efficacy and safety of oral WAYRILZ (400 mg BID) for 24 weeks, followed by a 28-week open-label extension (OLE) in adults with persistent or chronic ITP who had an insufficient response to either intravenous immunoglobulin (IVlg/anti-D) or corticosteroids (CS), or had a documented intolerance or insufficient response to any appropriate course of standard-of-care ITP therapy.1,2
Baseline demographics (N=202)1,2
| WAYRILZ (n=133) | Placebo Control (n=69) | |
| Median age (range) | 47 years (18-80) | 46 years (19-79) |
| Female, n (%) | 78 (59) | 49 (71) |
| Median duration of ITP (range) | 8.1 years (0.3-52.2) | 6.2 years (0.3-35.8) |
| Median baseline platelet count (range) | 15x109/L (1-32) | 15x109/L (1-54) |
| Prior splenectomy, n (%) | 37 (28) | 19 (28) |
| No concomitant treatment, n (%) | 53 (40) | 23 (33) |
| Concomitant CS, TPO-RA, or both, n (%) | 80 (60) | 46 (67) |
Prior unique ITP medications (pooled)2†
| Number of Medications | Percentage of Patients (N=202) |
| 1-2 | 29% (n=58) |
| 3-4 | 25% (n=51) |
| ≥5 | 46% (n=93) |
*Including those with insufficient response to corticosteroids and/or multiple other treatments.1,2
†Includes splenectomy. Multiple CS were counted as 1 therapy.2
Primary endpoint1,2
- Durable platelet response at Week 25
Additional endpoints1,2
- Time to first platelet response
- Number of weeks with platelet response
- Percentage of patients who needed rescue therapy
Patients with prior TEs were not excluded from the study population.2
Patient Disposition2
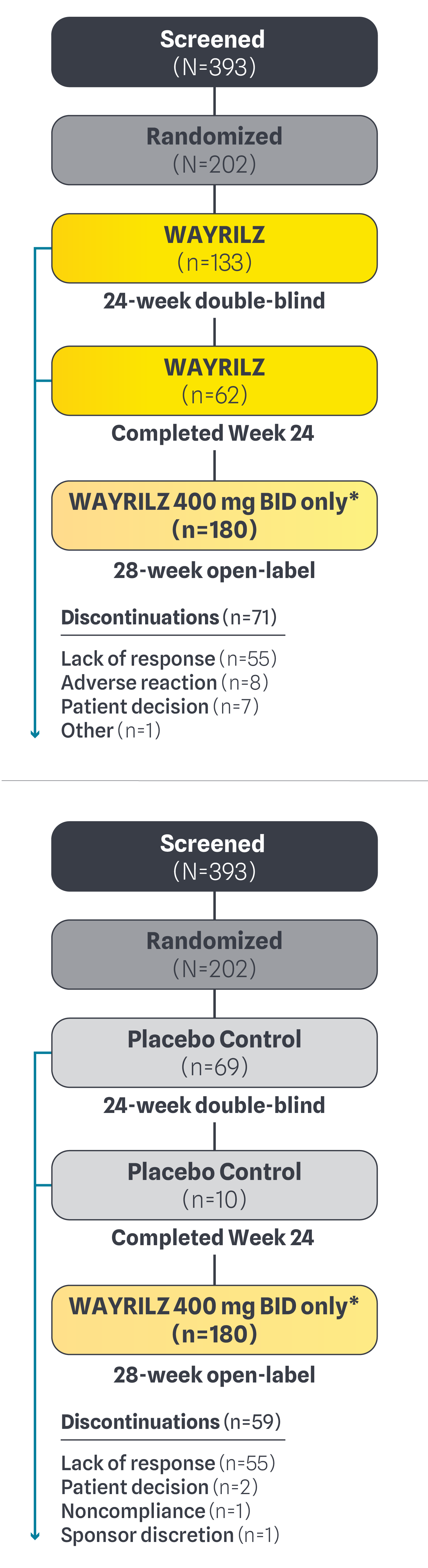
*Includes patients who entered as of the data cutoff. All patients in the 28-week open-label received WAYRILZ 400 mg BID.2
WAYRILZ: LUNA-3 study design1-3

Response Criteria
To qualify for continuation in the study through the end of the
DB period, patients had to have ≥1 platelet response of at least
50x109/L or ≥30x109/L and <50x109/L and at least doubled
from baseline by Week 13 without rescue therapy.1,2*
PRIMARY ENDPOINT: Durable platelet response
A platelet count ≥50x109/L for ≥5 of at least 8 non-missing weekly measurements during the last 12 weeks of the DB period, including ≥2 such responses in the last 6 weeks without rescue therapy.1*

At Week 13, 64% of patients receiving WAYRILZ were responders (n=85) compared to 32% of patients in the placebo control group (n=22).1,2
*Rescue therapy= intravenous immunoglobulin (IVlg), high-dose CS, platelet infusion, or anti-D immunoglobulin infusion (anti-D).
WAYRILZ was studied in a wide range of patients1,2*
LUNA-3: A phase 3, randomized, double-blind, placebo-controlled, parallel-group, multicenter study to evaluate the efficacy and safety of oral WAYRILZ (400 mg BID) for 24 weeks, followed by a 28-week open-label extension (OLE) in adults with persistent or chronic ITP who had an insufficient response to either intravenous immunoglobulin (IVlg/anti-D) or corticosteroids (CS), or had a documented intolerance or insufficient response to any appropriate course of standard-of-care ITP therapy.1,2
Baseline demographics (N=202)1,2
| WAYRILZ (n=133) | Placebo Control (n=69) | |
| Median age (range) | 47 years (18-80) | 46 years (19-79) |
| Female, n (%) | 78 (59) | 49 (71) |
| Median duration of ITP (range) | 8.1 years (0.3-52.2) | 6.2 years (0.3-35.8) |
| Median baseline platelet count (range) | 15x109/L (1-32) | 15x109/L (1-54) |
| Prior splenectomy, n (%) | 37 (28) | 19 (28) |
| No concomitant treatment, n (%) | 53 (40) | 23 (33) |
| Concomitant CS, TPO-RA, or both, n (%) | 80 (60) | 46 (67) |
Prior unique ITP medications (pooled)2†
| Number of Medications | Percentage of Patients (N=202) |
| 1-2 | 29% (n=58) |
| 3-4 | 25% (n=51) |
| ≥5 | 46% (n=93) |
*Including those with insufficient response to corticosteroids and/or multiple other treatments.1,2
†Includes splenectomy. Multiple CS were counted as 1 therapy.2
Primary endpoint1,2
- Durable platelet response at Week 25
Additional endpoints1,2
- Time to first platelet response
- Number of weeks with platelet response
- Percentage of patients who needed rescue therapy
Patients with prior TEs were not excluded from the study population.2
Patient Disposition2

*Includes patients who entered as of the data cutoff. All patients in the 28-week open-label received WAYRILZ 400 mg BID.2
anti-D, anti-D immunoglobulin; BID, twice daily; CS, corticosteroids; DB, double-blind; ITP, immune thrombocytopenia; IVIg, intravenous immunoglobulin; MOA, mechanism of action; TE, thromboembolic event; TPO-RA, thrombopoietin receptor agonist.
INDICATION
References: 1. WAYRILZ. Prescribing information. Sanofi, Inc. 2. Kuter DJ, Ghanima W, Cooper N, et al; LUNA3 Trial Group. Safety and efficacy of rilzabrutinib vs placebo in adults with immune thrombocytopenia: the phase 3 LUNA3 study. Blood. 2025;145(24):2914-2926. 3. Kuter DJ, Bussel JB, Ghanima W, et al. Rilzabrutinib versus placebo in adults and adolescents with persistent or chronic immune thrombocytopenia: LUNA 3 phase III study. Ther Adv Hematol. 2023;14:20406207231205431.