ITP is a disease of complex immune dysregulation1-3
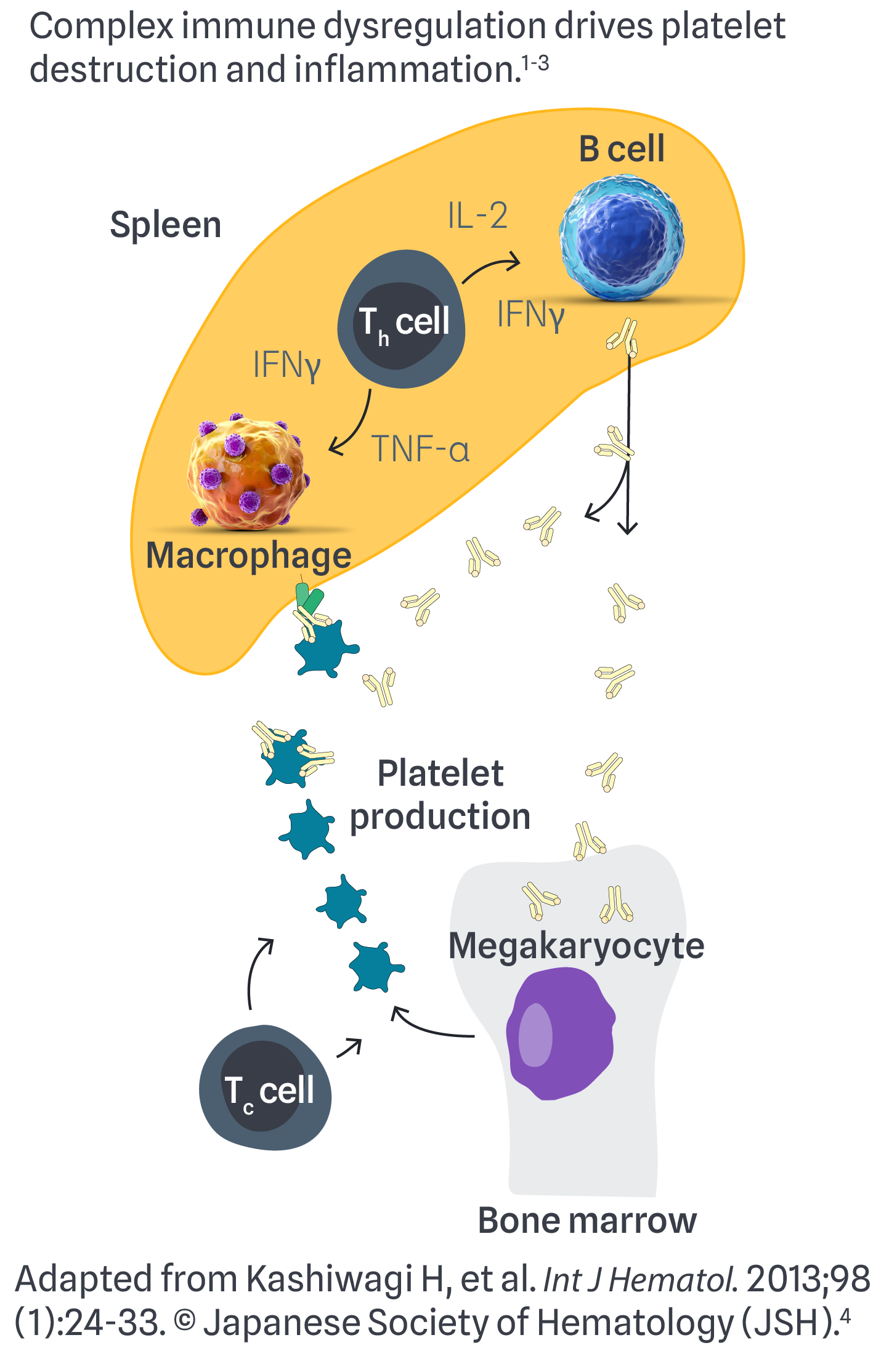
B cells produce an abundance of antiplatelet autoantibodies, leading to the destruction of platelets via phagocytosis by macrophages and impaired thrombopoiesis1-3
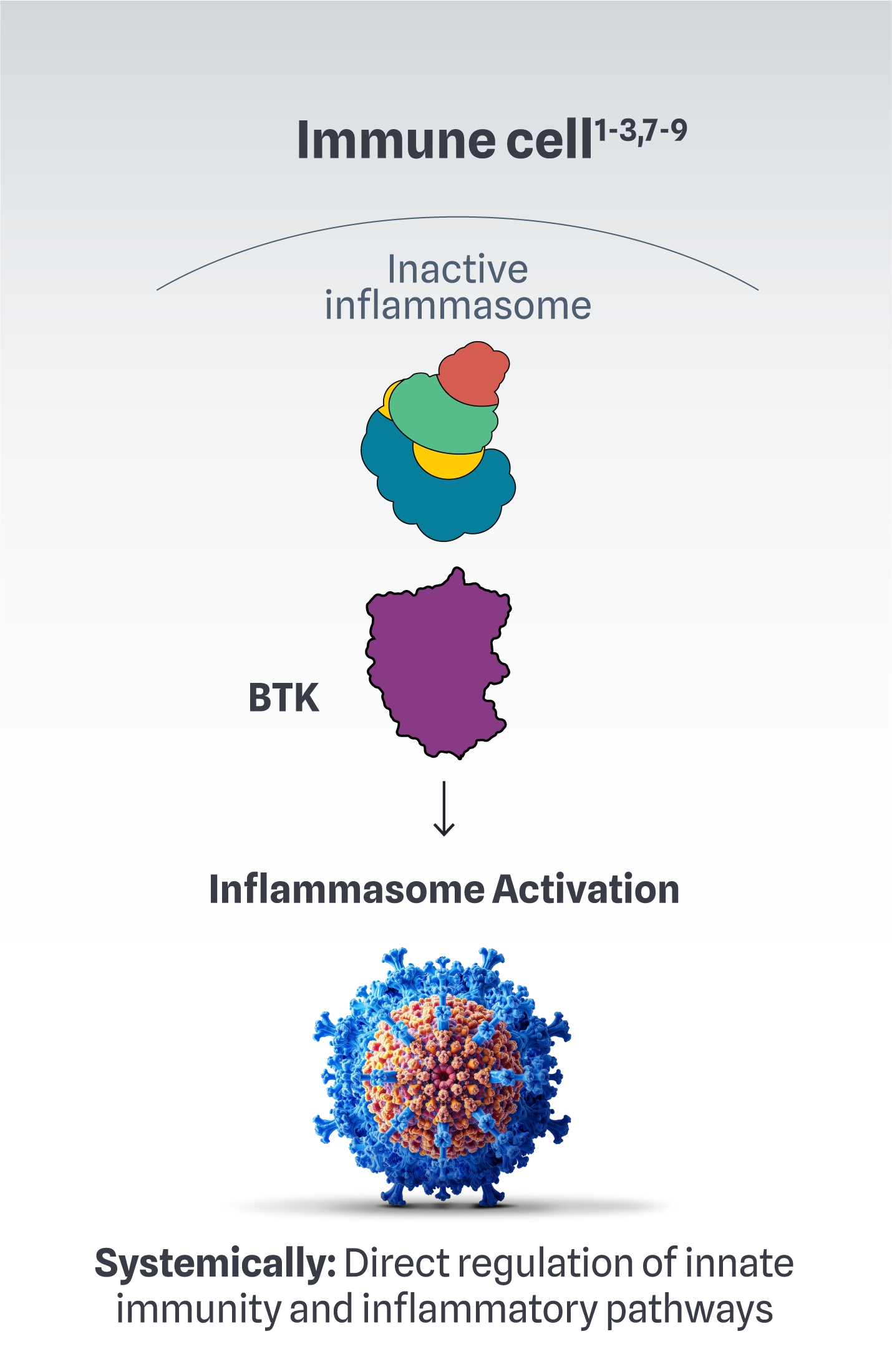
Inflammation occurs via an imbalance of proinflammatory factors (eg, IFNγ, TNF-α) and dysregulation of T cells. This is further amplified by increased NLRP3 inflammasome expression in other immune cells1-3

Treatments designed to achieve multi-immune modulation aim to better address the complex immune dysregulation of ITP1-3,5,6
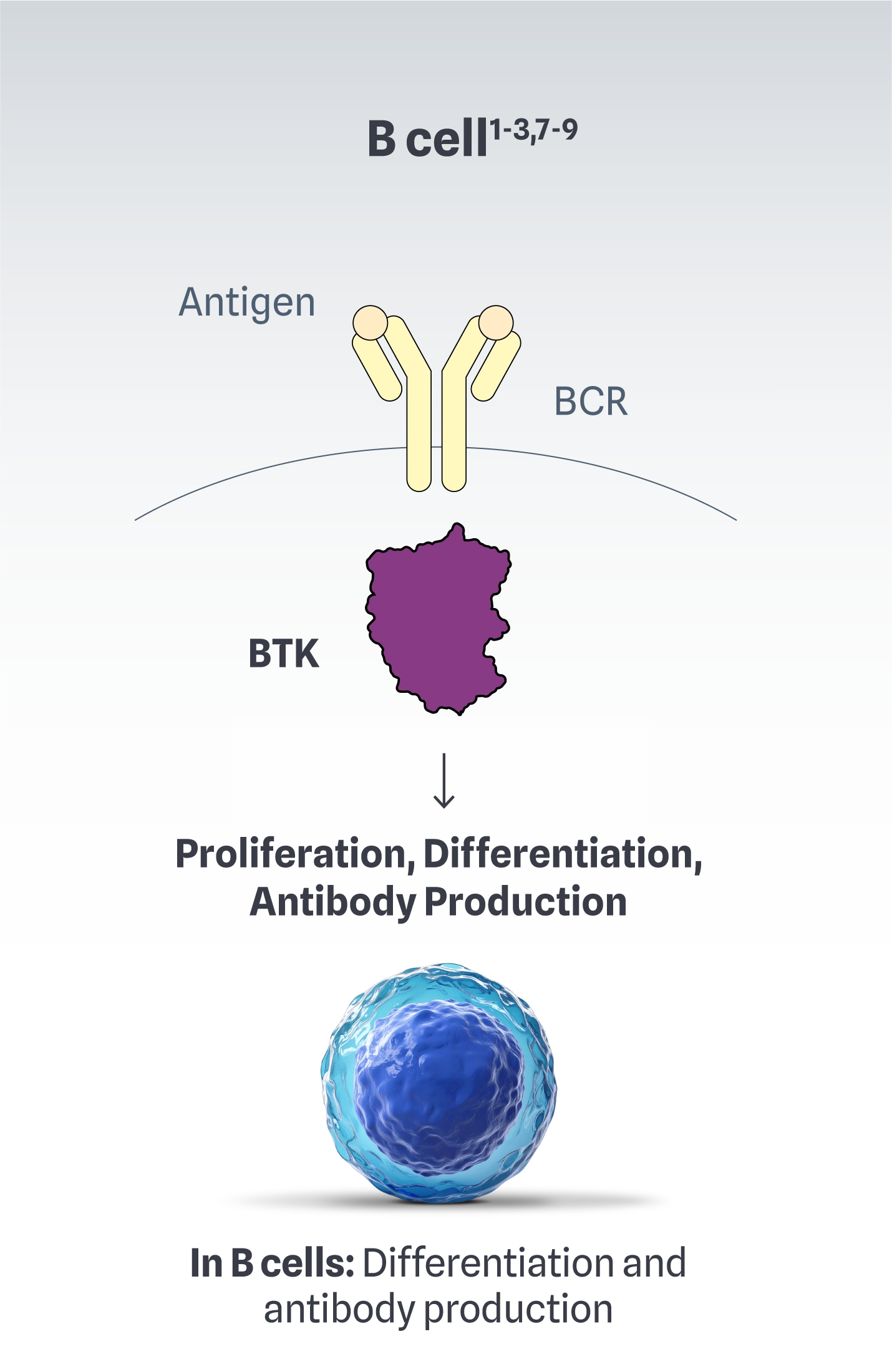
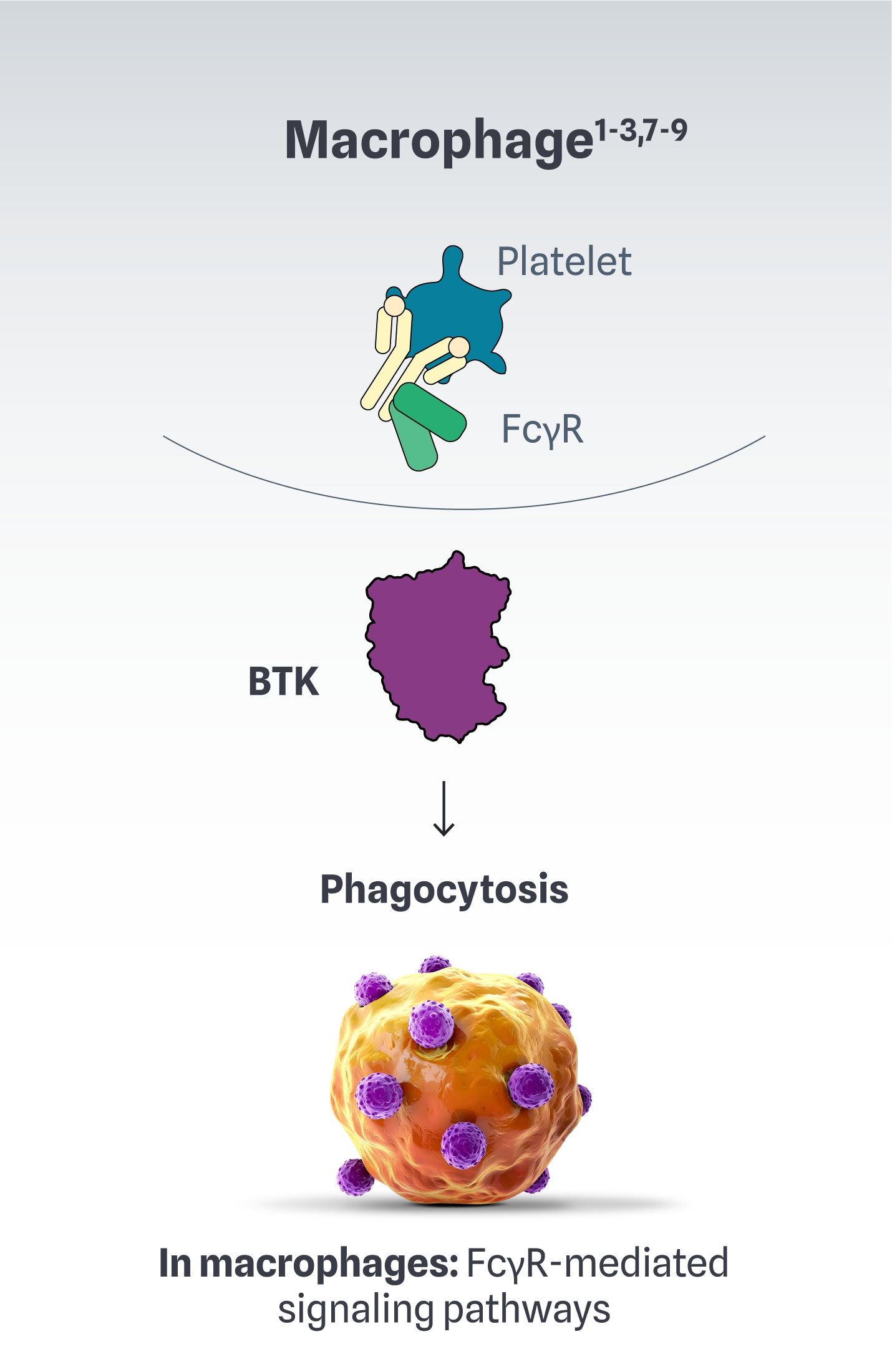
BTK is at the source of complex immune dysregulation1-3,7-9
In autoimmune conditions, BTK has a role in the following immune cell functions1-3,7-9:
BCR, B-cell receptor; BTK, Bruton's tyrosine kinase; BTKi, Bruton's tyrosine kinase inhibitor; FcγR, Fe gamma receptor; IFN, interferon; IL, interleukin; ITP, immune thrombocytopenia; MOA, mechanism of action; NLRP3, nucleotide-binding domain, leucine-rich repeat and pyrin domain containing protein 3; Tc, cytotoxic T [cell]; Th, helper T [cell]; TNF, tumor necrosis factor.
INDICATION
References: 1. Andreescu M. The link between immune thrombocytopenia and the cytokine profile: a bridge to new therapeutical targets. Front Hematol. 2023;2:1191178. 2. Qiao J, Liu Y, Li X, et al. Elevated expression of NLRP3 in patients with immune thrombocytopenia. Immunol Res. 2016;64(2):431-437. 3. Schifferli A, Cavalli F, Godeau B, et al. Understanding immune thrombocytopenia: looking out of the box. Front Med (Lausanne). 2021;8:613192. 4. Kashiwagi H, Tomiyama Y. Pathophysiology and management of primary immune thrombocytopenia. Int J Hematol. 2013;98(1):24-33. 5. Audia S, Bonnotte B. Emerging therapies in immune thrombocytopenia. J Clin Med. 2021;10(5):1004. 6. Mingot-Castellano ME, Bastida JM, Caballero-Navarro G, et al. Novel therapies to address unmet needs in ITP. Pharmaceuticals (Basel). 2022;15(7):779. 7. Zhu S, Gokhale S, Jung J, et al. Multifaceted immunomodulatory effects of the BTK inhibitors ibrutinib and acalabrutinib on different immune cell subsets – beyond B lymphocytes. Front Cell Dev Biol. 2021; 9:727531. 8. Neys SFH, Hendriks RW, Corneth OBJ. Targeting Bruton's tyrosine kinase in inflammatory and autoimmune pathologies. Front Cell Dev Biol. 2021;9:668131. 9. Kuter DJ, Bussel JB, Ghanima W, et al. Rilzabrutinib versus placebo in adults and adolescents with persistent or chronic immune thrombocytopenia: LUNA 3 phase III study. Ther Adv Hematol. 2023;14:20406207231205431. 10. WAYRILZ. Prescribing information. Sanofi, Inc.