- Article
- Source: Campus Sanofi
- 21 Jul 2025
Patient morbidity and mortality in BP

Mortality can be more than three times higher in patients with bullous pemphigoid compared with the age-matched general population1
The mortality of bullous pemphigoid
The 1-year mortality rate ranges from 20 to 40% in patients with bullous pemphigoid1–5
In a systematic review and meta-analysis of observational studies, a 3.4-fold increased mortality was observed among patients with bullous pemphigoid compared with the age-matched general population1
A Kaplan-Meier curve depicting survival in patients with bullous pemphigoid vs general population. Patients with bullous pemphigoid have a 3.4-fold increased mortality (N=287)a 1
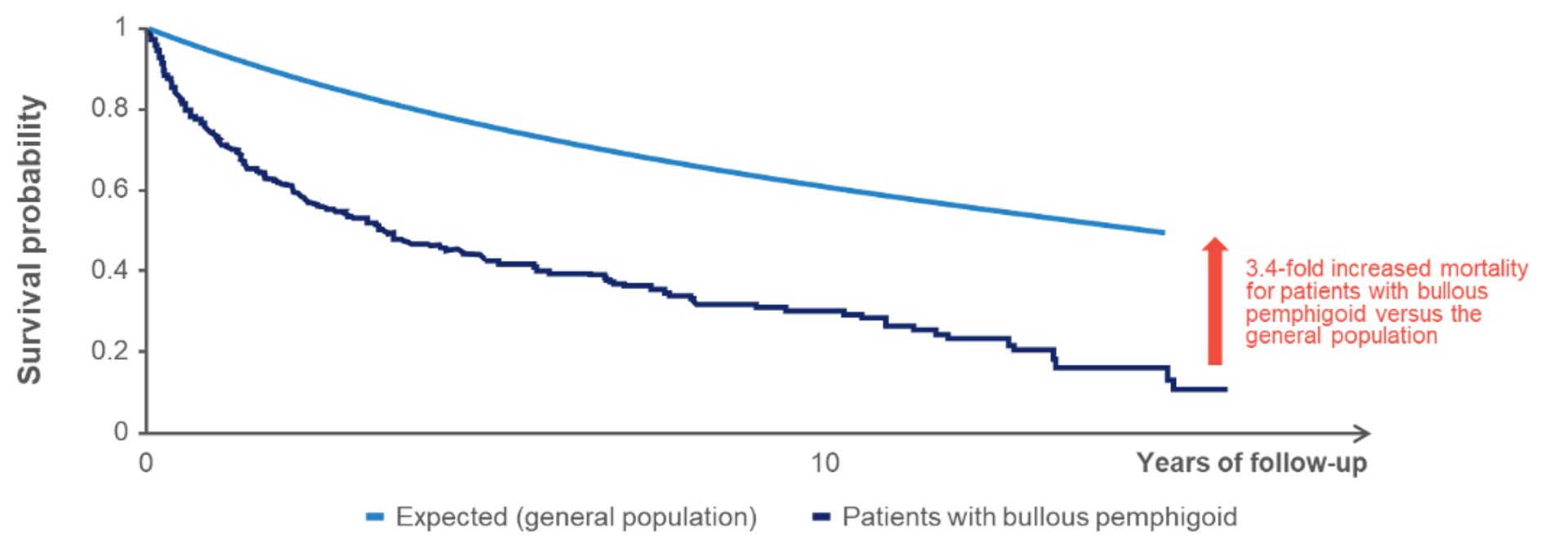
Figure adapted from Kridin K, et al. Acta Derm Venereol. 2019
aRetrospective cohort study comparing mortality in patients with bullous pemphigoid (N=287) diagnosed between 2000 and 2015 in Israel versus age- and sex-matched controls.1
Prognostic factors associated with mortality in bullous pemphigoid6–11
Various factors are associated with poor disease outcomes in bullous pemphigoid:

Prognostic variables for mortality in bullous pemphigoid can include:
- Higher dosage of systemic corticosteroids/immunosuppressive drugs6
- Older age at onset and poor performance scale (Karnofsky) 6–9
- Low serum albumin levels6
- Multimorbidity and certain comorbidities (e.g. neurological, cardiac, and renal comorbidities)8,10,11

High serum anti-BP180 IgG levels are associated with increased 1-year mortality rates in bullous pemphigoid12,13
Additionally, autoantibody profiles may help predict bullous pemphigoid disease course and occurrence of relapse12,14,15
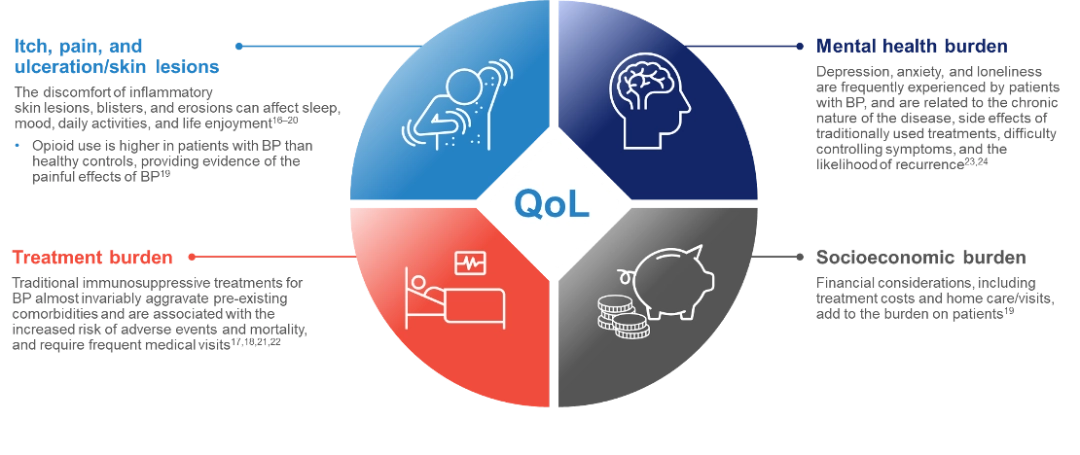
The impact of bullous pemphigoid on patients’ lives
Bullous pemphigoid often leads to substantial physical discomfort and limitations from severe itch and painful eczematous lesions, blisters, and erosions.16–21 This can result in mental, emotional, and socioeconomic burdens and severely impair patients’ quality of life.19, 22–24
-
Kridin K, et al. Acta Derm Venereol. 2019;99(1):72–7.
-
Schmidt E and Zillikens D. Lancet. 2013;381(9863):320–32.
-
Joly P, et al. J Invest Dermatol. 2012;132(8):1998–2004.
-
Tedbirt B, et al. JAMA Dermatol. 2021;157(4):421–30.
-
Cortés B, et al. Br J Dermatol. 2011;165:368–74.
-
Rzany B, et al. Arch Dermatol. 2002;138(7):903–8.
-
Liu YD, et al. Arch Dermatol Res. 2017;309(5):335–47.
-
Bardazzi F, et al. J Eur Acad Dermatol Venereol. 2022;36(12):2473–81.
-
Joly P et al Arch Dermatol. 2005;141(6):691–8.
-
Cai SCS, et al. Br J Dermatol. 2014;170(6):1319–26.
-
Papara C, et al. Indian J Dermatol Venereol Leprol. 2023;89(3):363–71.
-
Holtsche MM, et al. Br J Dermatol. 2018;179(4):918–24.
-
Bernard P, et al. BJD. 1997;136:694–698.
-
Fichel F, et al. JAMA Dermatol. 2014;150(1):25–33.
-
Bernard P, et al. Arch Dermatol. 2009;145(5):537–42.
-
Dan J, et al. J Am Acad Dermatol. 2023;89(1):159–60.
-
Lee J, et al. Front Immunol. 2019;10:2219.
-
Penha MA, et al. An Bras Dermatol. 2015;90:190–194.
-
Stirnadel-Farrant HA, et al. JAAD Int. 2023:13:117–25.
-
Sebaratnam DF, et al. Clin Dermatol. 2012;30:103–107.
-
Pratasava V, et al. Medicina (Kaunas). 2021;57:1061.
-
Han A. J Clin Aesthet Dermatol. 2009;2(5):19–28.
-
Kouris A, et al. An Bras Dermatol. 2016;91(5):601–3.
-
Hopkins ZH, et al. JAMA Dermatol. 2023;159:1185–1194.
-
Bernard P and Antonicelli F. Am J Clin Dermatol. 2017;18(4):513–28.
MAT-AE-2500459/V1/June 2025