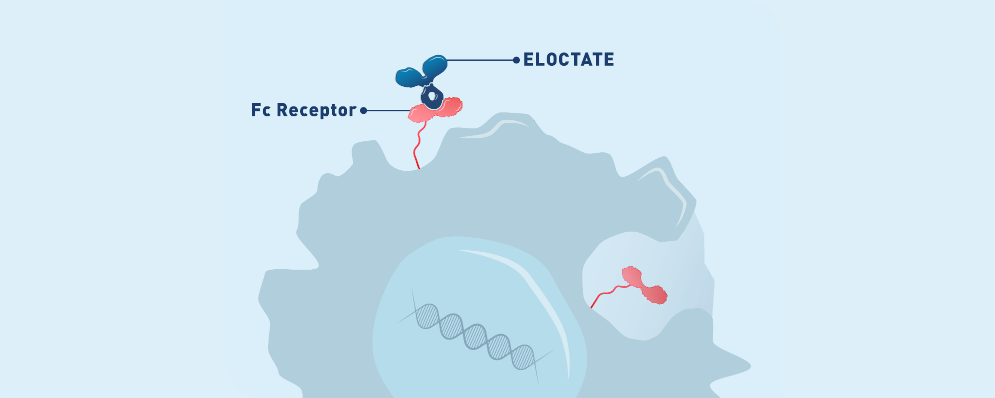
Fc Fusion MOE |
ELOCTATE contains the Fc region of IgG11
• IgG1 allows ELOCTATE to extend its half-life by binding to the neonatal Fc receptor1
• IgG1 is a naturally occurring protein that has anti-inflammatory and immunomodulatory properties2,3
• The molecular mechanism behind the effects achieved by ELOCTATE is not
yet fully understood, including the interaction with cellular Fc receptors that
ELOCTATE may interact with2
.png)
Fc=fragment crystallizable; FVIII=factor VIII; IgG1=immunoglobin G1; MOE=mechanism of extension.
INDICATION
References: 1. ELOCTATE [package insert]. Waltham, MA: Bioverativ Therapeutics Inc. 2. Kis-Toth K, et al. Blood Adv. 2018;2(21):2904-2916. 3. Kaneko Y, et al. Science. 2006;313(5787):670-673. 4. Shapiro A. Expert Opin Biol Ther. 2013;13(9):1287-1297.