Important Safety Information for Lantus (insulin glargine injection) 100 Units/mL
Contraindications
Lantus is contraindicated during episodes of hypoglycemia and in patients with hypersensitivity to insulin glargine or any of the excipients in LANTUS.
Warnings and Precautions
Insulin pens, needles, or syringes must never be shared between patients. Do NOT reuse needles.
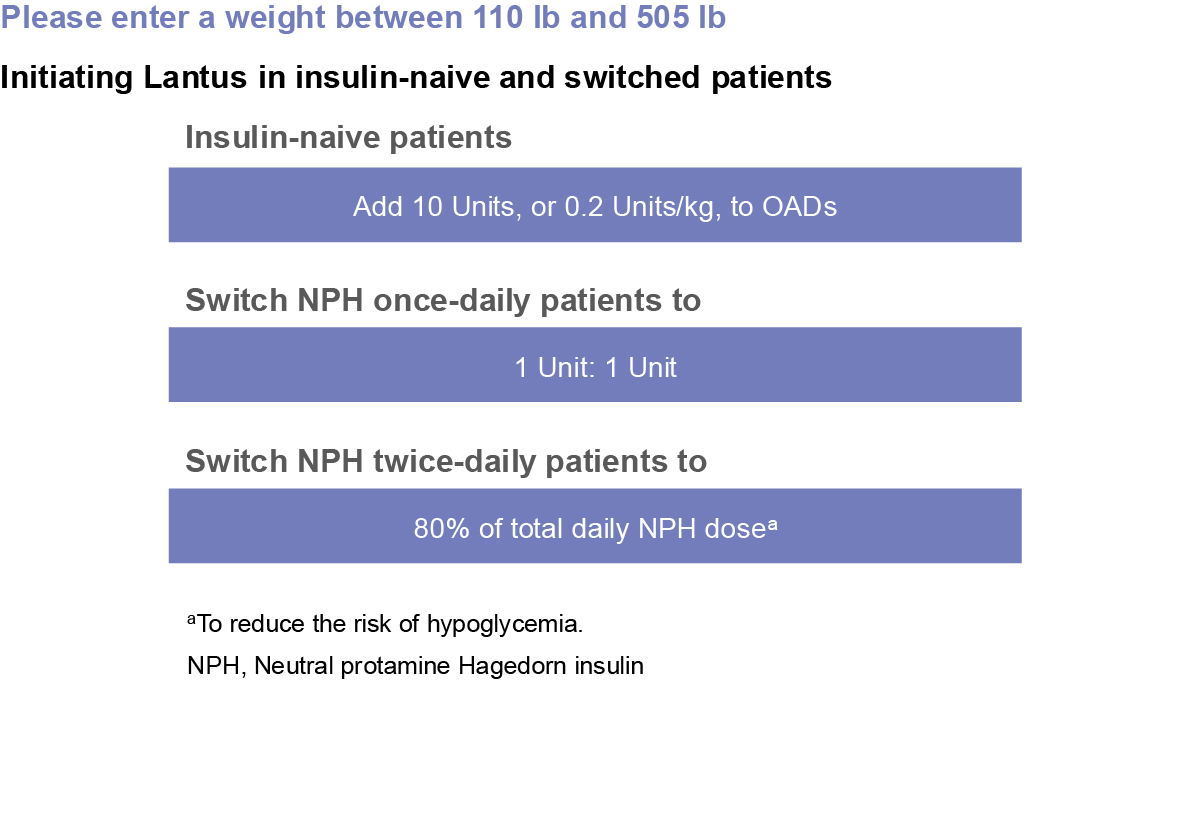
Monitor blood glucose in all patients treated with insulin. Modify insulin regimen only under medical supervision. Changes in insulin regimen including, strength, manufacturer, type, injection site or method of administration may result in the need for a change in insulin dose or an adjustment in concomitant drugs.
Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis may result in hyperglycemia; sudden change in the injection site (to unaffected area) has been reported to result in hypoglycemia. Advise patients to rotate injection site to unaffected areas and closely monitor for hypoglycemia.
Do not dilute or mix Lantus with any other insulin or solution. If mixed or diluted, the solution may become cloudy, and the onset of action/time to peak effect may be altered in an unpredictable manner. Do not administer Lantus via an insulin pump or intravenously because severe hypoglycemia can occur.
Hypoglycemia is the most common adverse reaction of insulin therapy, including Lantus, and may be life-threatening.
Hypoglycemia due to medication errors, such as accidental mix-ups between basal insulin products and other insulins, particularly rapid-acting insulins, have been reported. Patients should be instructed to always verify the insulin label before each injection.
Severe life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue Lantus, treat and monitor until symptoms resolve.
A reduction in the Lantus dose may be required in patients with renal or hepatic impairment.
As with all insulins, Lantus use can lead to life-threatening hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Closely monitor potassium levels in patients at risk of hypokalemia and treat if indicated.
Fluid retention, which may lead to or exacerbate heart failure, can occur with concomitant use of thiazolidinediones (TZDs) with insulin. These patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of TZD must be considered.
Drug Interactions
Certain drugs may affect glucose metabolism, requiring insulin dose adjustment and close monitoring of blood glucose. The signs of hypoglycemia may be reduced in patients taking anti-adrenergic drugs (e.g., beta-blockers, clonidine, guanethidine, and reserpine).
Adverse Reactions
Adverse reactions commonly associated with Lantus include hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, rash, edema and weight gain.
Important Safety Information for Lantus SoloSTAR
Lantus SoloSTAR is a disposable single-patient-use prefilled insulin pen. To help ensure an accurate dose each time, patients should follow all steps in the Instruction Leaflet accompanying the pen: otherwise they may not get the correct amount of insulin, which may affect their blood glucose.