.png)
Acquired/immune-mediated thrombotic thrombocytopenic purpura (aTTP/iTTP) is a rare, rapidly progressing, life-threatening medical emergency. It has been reported in literature that, when left untreated, death may occur in up to 90% of patients with aTTP/iTTP, making urgent diagnosis and treatment a necessity.1-6
Identifying aTTP/iTTP is crucial for initiation of an appropriate therapeutic strategy7
Diagnose aTTP/iTTP through clinical assessment or risk assessment tools prior to ADAMTS13 testing.
Clinical assessment7 | OR |
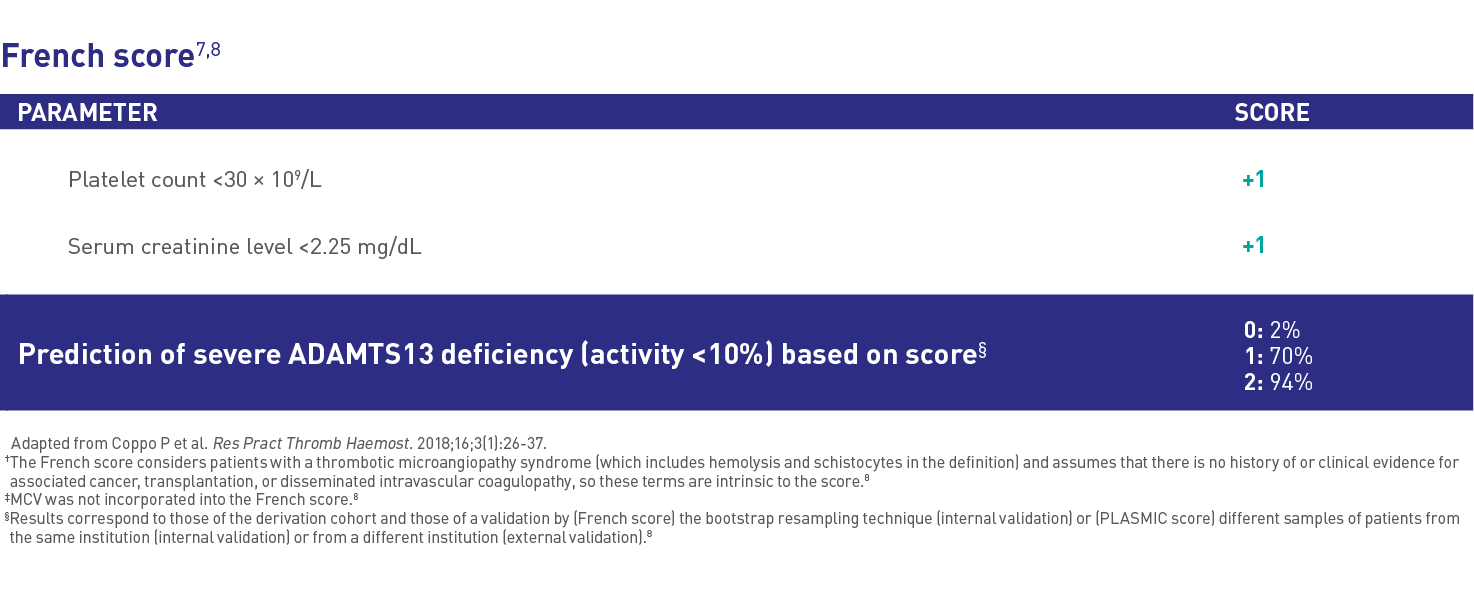
Risk assessment7* |
|
Patient presentation prompting suspicion of aTTP:
|
Available risk assessment tools include:
The higher the risk assessment score, the more likely patients have severe ADAMTS13 deficiency and aTTP/iTTP |
*ISTH did not appraise the evidence for these 2 tools.
Risk assessment tools can be used to predict ADAMTS13 deficiency

.png)
Complex presentation of TTP often causes a delay in diagnosis10
TTP presents with highly variable, multiorgan symptoms that resemble a series of thrombotic microangiopathy (TMAs) and other disorders. It is therefore critical to differentiate TTP from other similarly presenting conditions. The table below compares TTP with other resembling conditions.
Differentiating aTTP/iTTP from other similarly presenting TMAs11-18
| TTP | HUS | DIC | |
| Presenting symptoms |
• Thrombocytopenia (platelet count <150 × 109/L or >25% reduction from baseline)11,12 | ||
| Types | Congenital and acquired/immune-mediated TTP (cTTP and aTTP/iTTP)13,14 | Infection-associated or atypical/complement-mediated HUS (IA-HUS or aHUS/CM-HUS)14,15 | Overt and nonovert DIC16 |
| Age | cTTP—usually children14 aTTP/iTTP—usually adults14 | IA-HUS—common in children17 aHUS/CM-HUS—any age17 | NA |
| Cause | cTTP—inherited mutations of ADAMTS1314 aTTP/iTTP—autoantibodies against ADAMTS1314 | IA-HUS—toxins produced by certain bacteria17,18 aHUS/CM-HUS—activation of the complement system17,18 | Occurs as a result of underlying diseases such as sepsis, malignancy, trauma, liver diseases, obstetric disorders, envenomation, vascular anomalies, and major transfusion reactions16 |
| Potential laboratory results | Platelet count <30 × 109/L18 Creatinine <2.25 mg/dL18 PT and aPTT—normal11 D-dimer—normal11 ADAMTS13 <10%18 | Platelet count <30 × 109/L18 Creatinine >2.25 mg/dL18 PT and aPTT—normal11 D-dimer—normal11 ADAMTS13 ≥10%18 | Platelet count <50 × 109/L17 PT and aPTT—prolonged16,17 D-dimer—elevated16,17 |
| Test(s) confirming the diagnosis | cTTP—ADAMTS13 sequencing14,18 aTTP/iTTP—ADAMTS13 testing14,18 | IA-HUS—culture test, PCR, ADAMTS13 testing11,17 aHUS/CM-HUS—culture test, ADAMTS13 testing11,17 | ISTH scoring system for overt DIC16 |
Severe thrombocytopenia, MAHA, and organ ischemia indicate aTTP/iTTP is likely13
See what Guidelines suggest about treating as soon as you suspect aTTP/iTTP.
Is it TTP or aHUS?
The clinical presentation of aTTP/iTTP can be strikingly similar to that of aHUS, which is why diagnosis can often be complicated. The creatinine levels, platelet count, and ADAMTS13 levels may guide toward distinguishing one from the other.13,14
Differentiating TTP from HUS is crucial in order to start an appropriate therapy14
The following chart can help guide diagnosis to quickly differentiate patients with TTP in need of emergency care.
Adapted from: Kremer Hovinga JA et al. Nat Rev Dis Primers. 2017;3:17020.
Biopsies can be useful in differentiating aTTP/iTTP from aHUS in difficult cases19
Biopsies can be useful in difficult diagnostic situations. Sampling of any accessible, highly vascular site may help inform the differential diagnosis of a TMA, based on pathologic distinctions between aTTP/iTTP and aHUS.
aTTP/iTTP
Biopsy sample of any accessible, highly vascular site shows the following:
- Microthrombi appear as “white clots” composed of platelets and vWF, with only small amounts of fibrin
- Vascular or perivascular inflammatory cell infiltrations are minimal or absent
aHUS/CM-HUS
Biopsy sample of any accessible, highly vascular site shows the following:
- Microthrombi appear as “red clots,” predominated by fibrin
- An inflammatory infiltrate may be seen together with deposits of C5b-9
EHR capabilities such as Rule Messages can support proactive identification of at-risk patients for further evaluation to differentiate aTTP/iTTP from other conditions
ADAMTS13=a disintegrin and metalloproteinase with a thrombospondin type 1 motif, 13; aHUS=atypical hemolytic uremic syndrome; aPTT=activated partial thromboplastin time; CM-HUS=complement-mediated hemolytic uremic syndrome; cTTP=congenital thrombotic thrombocytopenic purpura; DIC=disseminated intravascular coagulation; EHR=electronic health record; Hb=hemoglobin; HUS=hemolytic uremic syndrome; IA-HUS=infection-associated hemolytic uremic syndrome; ISTH=International Society on Thrombosis and Haemostasis; LDH=lactate dehydrogenase; MAHA=microangiopathic hemolytic anemia; MCV=mean corpuscular volume; PCR=polymerase chain reaction; PT=prothrombin time; TTP=thrombotic thrombocytopenic purpura; vWF=von Willebrand factor.
INDICATION
References: 1. Scully M, Hunt BJ, Benjamin S, et al; British Committee for Standards in Haematology. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 2012;158(3):323-335. doi:10.1111/j.1365-2141.2012.09167.x 2. Goel R, King KE, Takemoto CM, Ness PM, Tobian AAR. Prognostic risk-stratified score for predicting mortality in hospitalized patients with thrombotic thrombocytopenic purpura: nationally representative data from 2007 to 2012. Transfusion. 2016;56(6):1451-1458. doi:10.1111/trf.13586 3. Grall M, Azoulay E, Galicier L, et al. Thrombotic thrombocytopenic purpura misdiagnosed as autoimmune cytopenia: causes of diagnostic errors and consequence on outcome. Experience of the French Thrombotic Microangiopathies Reference Centre. Am J Hematol. 2017;92(4):381-387. doi:10.1002/ajh.24665 4. Kremer Hovinga JA, Vesely SK, Terrell DR, Lämmle B, George JN. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010;115(8):1500-1511. doi:10.1182/blood-2009-09-243790 5. Peyvandi F, Scully M, Kremer Hovinga JA, et al. Caplacizumab reduces the frequency of major thromboembolic events, exacerbations and death in patients with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2017;15(7):1448-1452. doi:10.1111/jth.13716 6. Sayani FA, Abrams CS. How I treat refractory thrombotic thrombocytopenic purpura. Blood. 2015;125(25):3860-3867. doi:10.1182/blood-2014-11-551580 7. Zheng XL, Vesely SK, Cataland SR, et al. ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2020;18(10):2486-2495. doi:10.1111/jth.15006 8. Coppo P, Cuker A, George JN. Thrombotic thrombocytopenic purpura: toward targeted therapy and precision medicine. Res Pract Thromb Haemost. 2018;3(1):26-37. doi:10.1002/rth2.12160 9. Bendapudi PK, Hurwitz S, Fry A, et al. Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. Lancet Haematol. 2017;4(4):e157-e164. doi:10.1016/S2352-3026(17)30026-1 10. Gallan AJ, Chang A. A new paradigm for renal thrombotic microangiopathy. Semin Diagn Pathol. 2020;37(3):121-126. doi:10.1053/j.semdp.2020.01.002 11. Vincent J-L, Castro P, Hunt BJ, et al. Thrombocytopenia in the ICU: disseminated intravascular coagulation and thrombotic microangiopathies—what intensivists need to know. Crit Care. 2018;22(1):158. doi:10.1186/s13054-018-2073-2 12. Nguyen TC, Cruz MA, Carcillo JA. Thrombocytopenia-associated multiple organ failure and acute kidney injury. Crit Care Clin. 2015;31(4):661-674. doi:10.1016/j.ccc.2015.06.004 13. Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836-2846. doi:10.1182/blood-2016-10-709857 14. Chiasakul T, Cuker A. Clinical and laboratory diagnosis of TTP: an integrated approach. Hematology Am Soc Hematol Educ Program. 2018;2018(1):530-538. doi:10.1182/asheducation-2018.1.530 15. Wada H, Matsumoto T, Suzuki K, et al. Differences and similarities between disseminated intravascular coagulation and thrombotic microangiopathy. Throm J. 2018;16:14. doi:10.1186/s12959-018-0168-2 16. Venugopal A. Disseminated intravascular coagulation. Indian J Anaesth. 2014;58(5):603-608. doi:10.4103/0019-5049.144666 17. Canpolat N. Hemolytic uremic syndrome. Turk Pediatri Ars. 2015;50(2):73-82. doi:10.5152/tpa.2015.2297 18. Kremer Hovinga JA, Coppo P, Lämmle B, Moake JL, Miyata T, Vanhoorelbeke K. Thrombotic thrombocytopenic purpura. Nat Rev Dis Primers. 2017;3:17020. doi:10.1038/nrdp.2017.20 19. Laurence J, Haller H, Mannucci PM, Nangaku M, Praga M, de Cordoba SR. Atypical hemolytic uremic syndrome (aHUS): essential aspects of an accurate diagnosis. Clin Adv Hematol Oncol. 2016;14(11)(suppl 11):2-15.
.png)