Results from Phase 3 Pivotal Study: HERCULES2,3
- Pivotal, phase 3, double-blind, randomized control trial of 145 adult patients with aTTP/iTTP
- Patients were given PEX and immunosuppressive therapy in the form of corticosteroid treatment. Other immunosuppressive treatments, such as rituximab, were permitted but not required*
- Patients were then randomized to receive CABLIVI® (n=72) or placebo (n=73) for the duration of daily PEX and 30 days thereafter
- Patients could receive extended treatment for up to 28 days if signs of underlying disease persisted, such as suppressed ADAMTS13 activity levels
- Primary endpoint: Time to platelet count normalization†
- Secondary endpoint: Composite of aTTP/iTTP-related events during study-drug period: aTTP/iTTP-related death, recurrence,‡ ≥1 major TE event
Compared with PEX and immunosuppressive therapy alone, patients receiving the addition of CABLIVI achieved2,3:
.png)
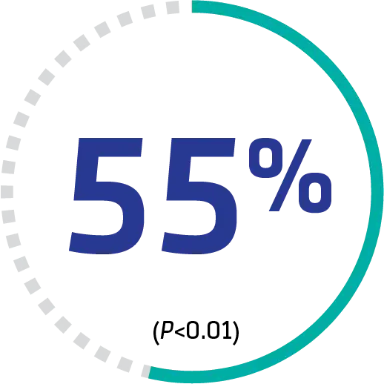
SIGNIFICANTLY FASTER time to platelet normalization†
HR (95% CI): 1.55 (1.10-2.20). Median days: 2.69 vs 2.88, respectively
.png)
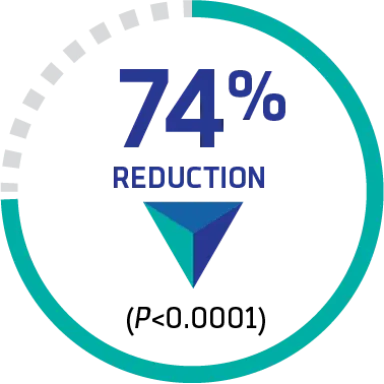
A SIGNIFICANT REDUCTION in a composite endpoint of aTTP/iTTP-related death, recurrence,‡ or ≥1 major TE event during treatment
9 (12.7%) vs 36 (49.3%), respectively
*2 patients in each group did not receive any immunosuppressive therapy.
†Platelet count normalization was defined as platelet count ≥150,000/μL, with discontinuation of daily PEX within 5 days thereafter.
‡Thrombocytopenia after initial recovery of platelet count (platelet count ≥150,000/μL) that required reinitiation of daily PEX was considered a recurrence. Recurrences were termed exacerbations if they occurred within 30 days of the last PEX and relapses if they occurred more than 30 days after the last PEX.
Real-world evidence supports CABLIVI® (caplacizumab‑yhdp) early use in over 1000 patients with aTTP/iTTP1
The Capla 1000+ Project is an unprecedented, independent, retrospective cohort study reporting on the largest international series of patients with aTTP/iTTP treated with CABLIVI (n=1015).1
The Capla 1000+ Project study design1
Study objective:
- To help assess whether CABLIVI decreases mortality and the optimal timing of CABLIVI initiation, a multi-institutional study was conducted
Study design:
- International, multicenter, independent, retrospective cohort study in which 1015 patients were treated primarily between 2018 and 2023 (98.7%) with daily PEX, immunosuppressive therapy with corticosteroids ± rituximab,§ and CABLIVI (CABLIVI group), which was compared with historic controls treated with PEX and corticosteroids ± rituximab (control group, n=510)
- CABLIVI initiation was classified as early (within 3 days; 76% of cases) or delayed (≥4 days from first PEX)
- Featured participation of expert clinicians and biologists from 12 university hospitals worldwide
- 2 cohorts of patients treated with CABLIVI and control patients were recruited on the basis of stringent inclusion criteria according to the updated definition of thrombotic thrombocytopenic purpura—ie, thrombotic microangiopathy with no associated condition and with severe ADAMTS13 deficiency
Study limitations:
- Due to the limitations of this study’s retrospective, multicenter, real-world analysis design, the findings represent associations and should not be interpreted as establishing causality
- The selection of patients involved only experienced teams, as reflected by the low death rate in both therapeutic groups; hence, our results may only apply to participating centers
- The use of rituximab was variable between teams and its use was more systematic in the CABLIVI group, reflecting a wider adoption of rituximab in the more-recent patients; however, virtually all patients in the CABLIVI group achieved clinical response within 8 days, compared with the previous 2 to 3 weeks onset of ADAMTS13 response observed with rituximab
Dosing administration in the phase 3 HERCULES clinical trial:
- CABLIVI was administered as a single 11-mg IV bolus injection prior to PEX,∥ followed by a daily 11-mg SC injection for the duration of PEX, and continued for 30 days thereafter1
§Rituximab is not approved by the US Food and Drug Administration (FDA) for the use under investigation. Sanofi does not promote or encourage the use of its products outside their FDA approved labeling.4
∥In combination with PEX and immunosuppressive therapy.
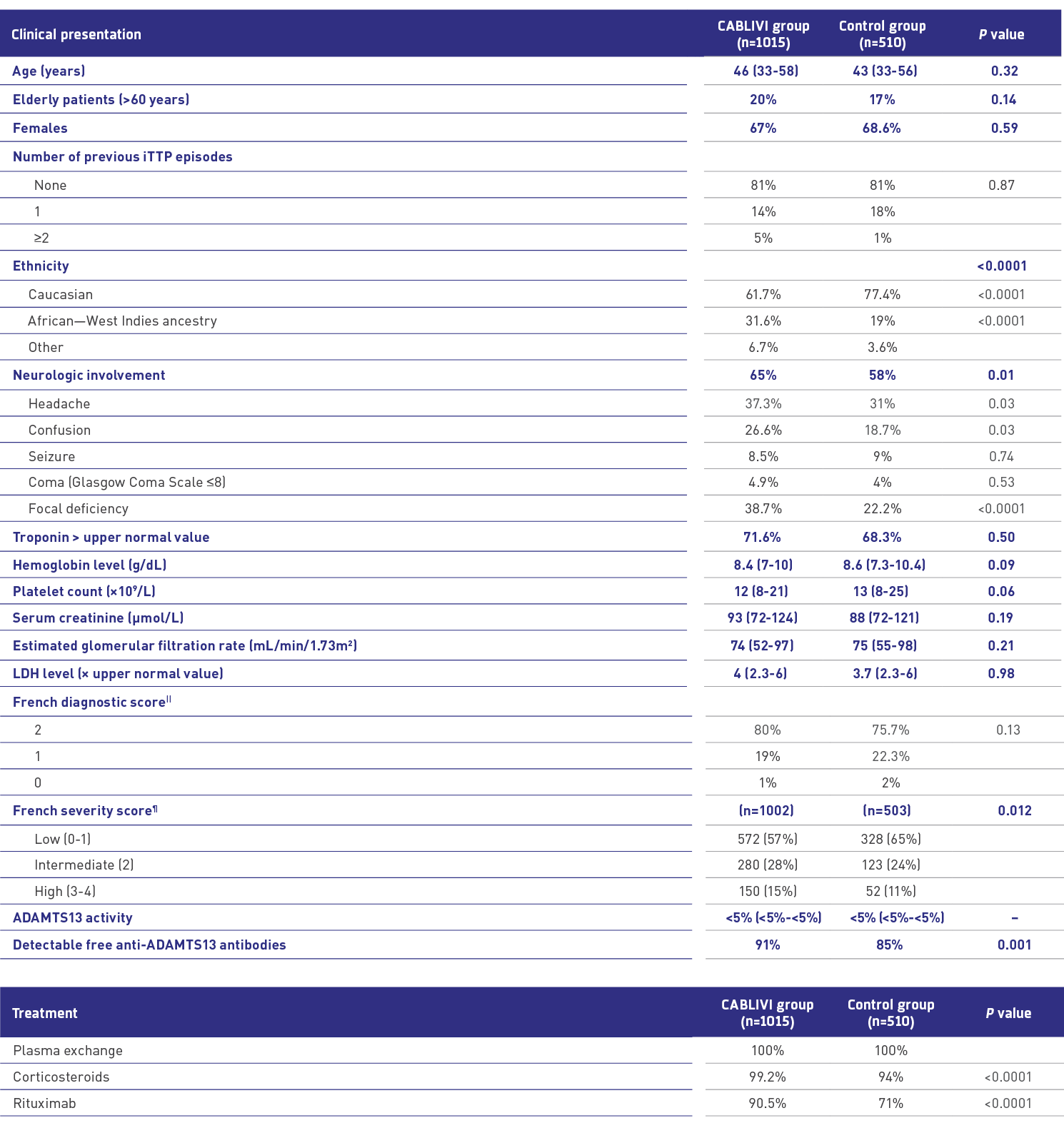
Comparable clinical presentation at diagnosis: CABLIVI vs control1
.png)
||French diagnostic score of 2: platelet count <30×103/mm3 and serum creatinine <200 μmol/L (2.27 mg/dL); score of 1: platelet count <30×103/mm3 or serum creatinine <200 μmol/L (2.27 mg/dL); score of 0: platelet count ≥30×103/mm3 and serum creatinine ≥200 μmol/L (2.27 mg/dL).
¶Patients at high risk of early death from aTTP were defined by French severity score ≥3 (cerebral involvement: yes=1/no=0; LDH: >10×ULN=1/≤10×ULN=0; age: >60 years=2/>40 and ≤60 years=1/≤40 years=0).
Study findings: Early CABLIVI use upon diagnosis and decision to start PEX
Findings from the Capla 1000+ Project “strongly argue for the systematic use of CABLIVI early in the management of aTTP/iTTP, typically as soon as the diagnosis and decision to start PEX is made, to prevent the formation of deleterious microthrombi and potentially prevent adverse outcomes.”1
—Investigators for the Capla 1000+ Project
Primary endpoint
3-month survival rate in CABLIVI group compared with control group1#
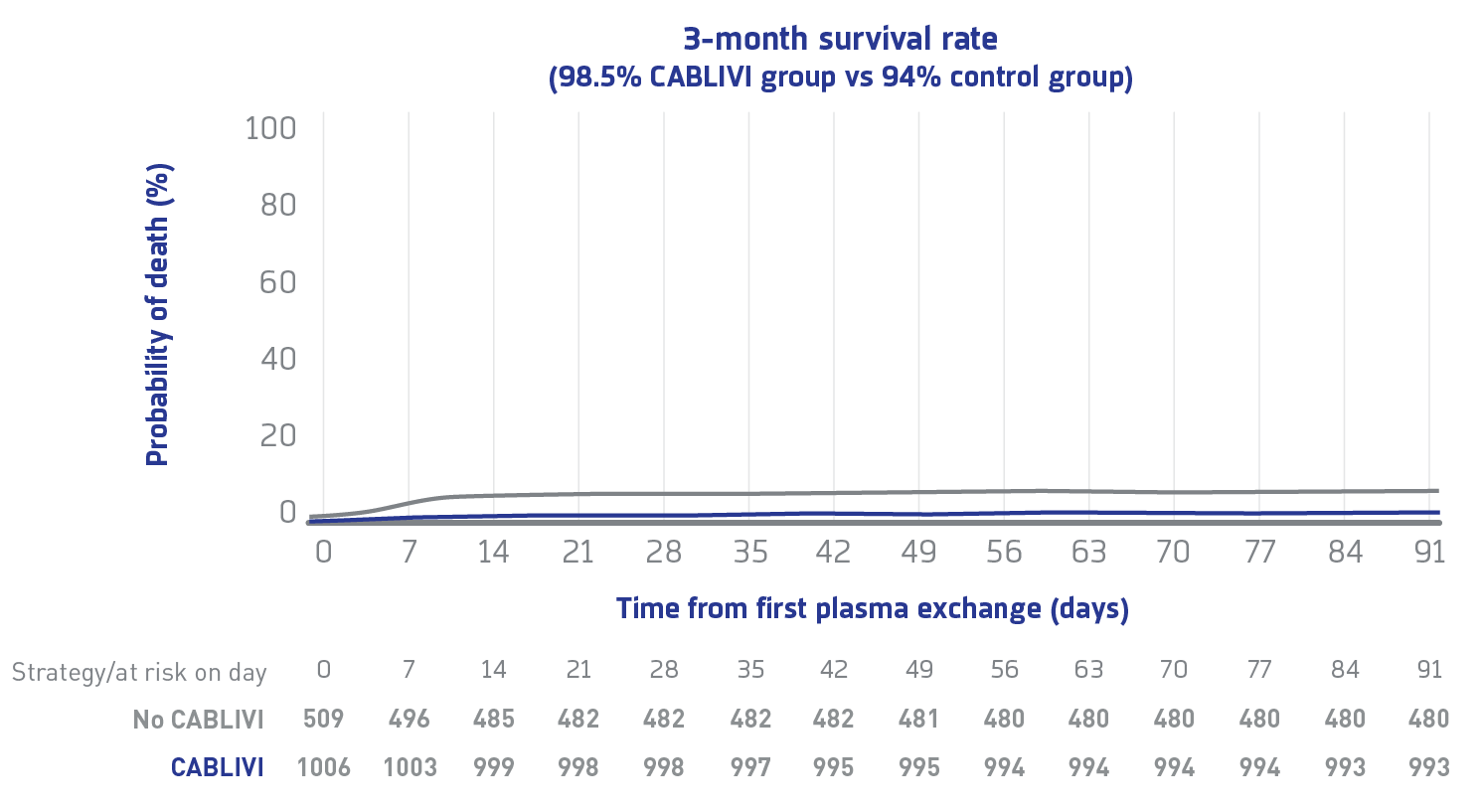
Due to the limitations of this study's retrospective, multicenter, real-world analysis design, the findings represent associations and should not be interpreted as establishing causality.
3-month mortality rate was 4.2-fold higher in controls vs the CABLIVI group (95% CI: 2.22-7.7), regardless of rituximab use.
Early vs delayed use
Time to clinical response in early CABLIVI group compared with control group1**
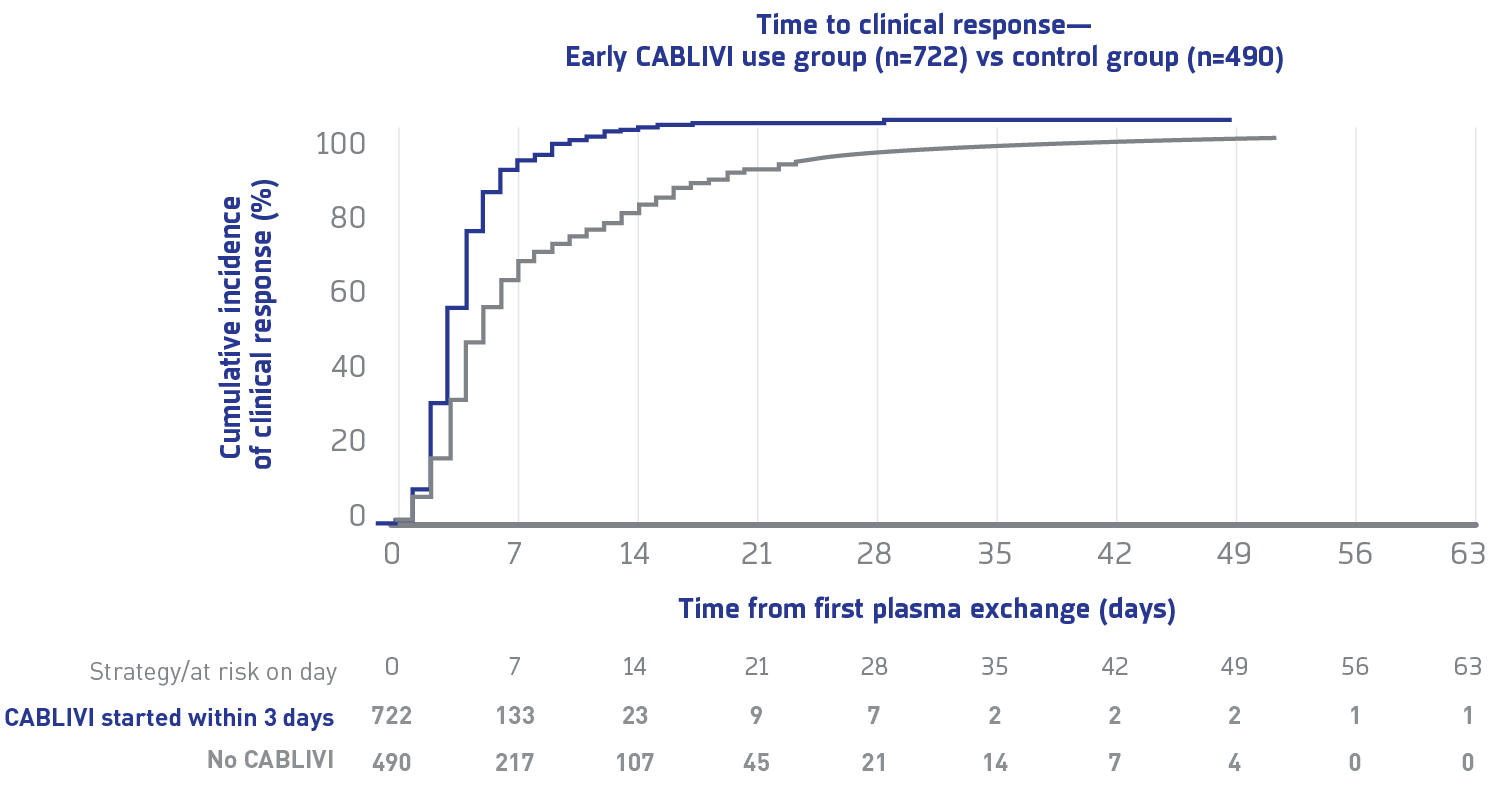
Due to the limitations of this study's retrospective, multicenter, real-world analysis design, the findings represent associations and should not be interpreted as establishing causality.
Median time to clinical response in the early CABLIVI group was 5 days (4-8) vs 6 days (4-12) in the control group.
Improved prognosis
Exacerbation rate, refractoriness, and number of PEX to achieve clinical response in CABLIVI group compared with control group1††,‡‡
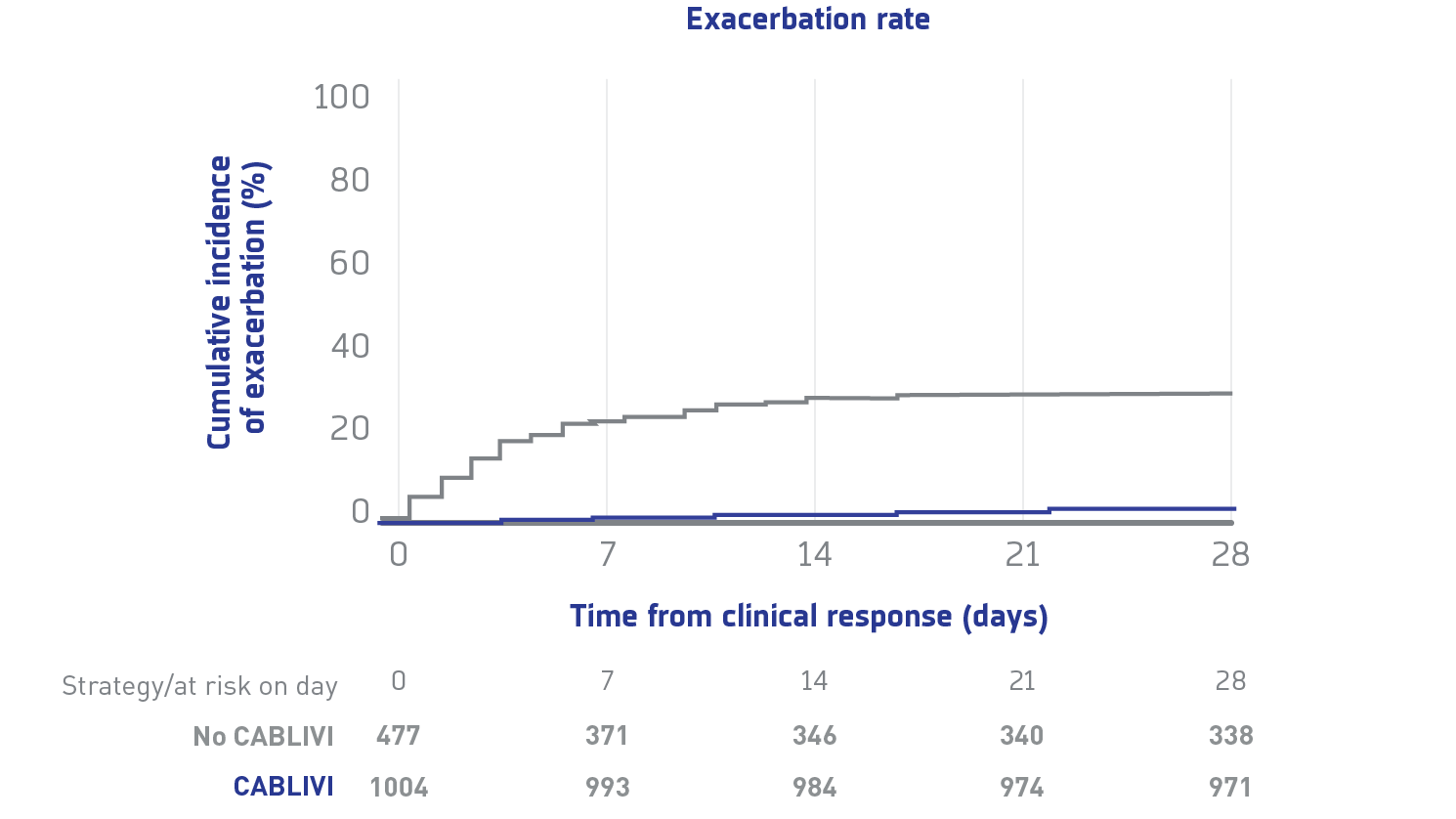
Due to the limitations of this study's retrospective, multicenter, real-world analysis design, the findings represent associations and should not be interpreted as establishing causality.
.png)
Exacerbations
Exacerbations were less frequent with CABLIVI vs the control group
.png)
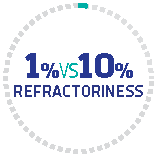
Refractoriness
Patients receiving CABLIVI experienced less-frequent refractoriness vs the control group (1% vs 10.1%)
.png)
Number of PEX
The CABLIVI group required fewer plasma exchanges to achieve clinical response (5 [4-8] vs 7 [4-16]), leading to an observed reduction in burden of care
#Death in the CABLIVI group (n=15/1015) was considered as directly related to uncontrolled aTTP/iTTP in 8 cases (53%); in 4 others, death resulted from aTTP/iTTP-related comorbidities, including 1 case of fatal intracerebral hemorrhage late in the management, while aTTP/iTTP was in clinical response; in the 3 remaining patients, death was not related to aTTP/iTTP. In the control group, death was directly related to uncontrolled aTTP/iTTP in all 26 patients with available data.
**In patients with delayed CABLIVI initiation, clinical response was obtained after a median of 4 days from CABLIVI initiation.
††Refractoriness was defined as persistent thrombocytopenia (considered severe if no doubling of baseline platelet count or platelet count <30x109/L; usually needs salvage therapy) by 4 days of standard treatment, together with persistently elevated LDH levels.
‡‡Exacerbation was defined as reappearance of thrombocytopenia, with or without clinical evidence of new ischemic organ injury within 30 days of stopping PEX or CABLIVI, with need to restart treatment.
Safety
Adverse events related to CABLIVI1
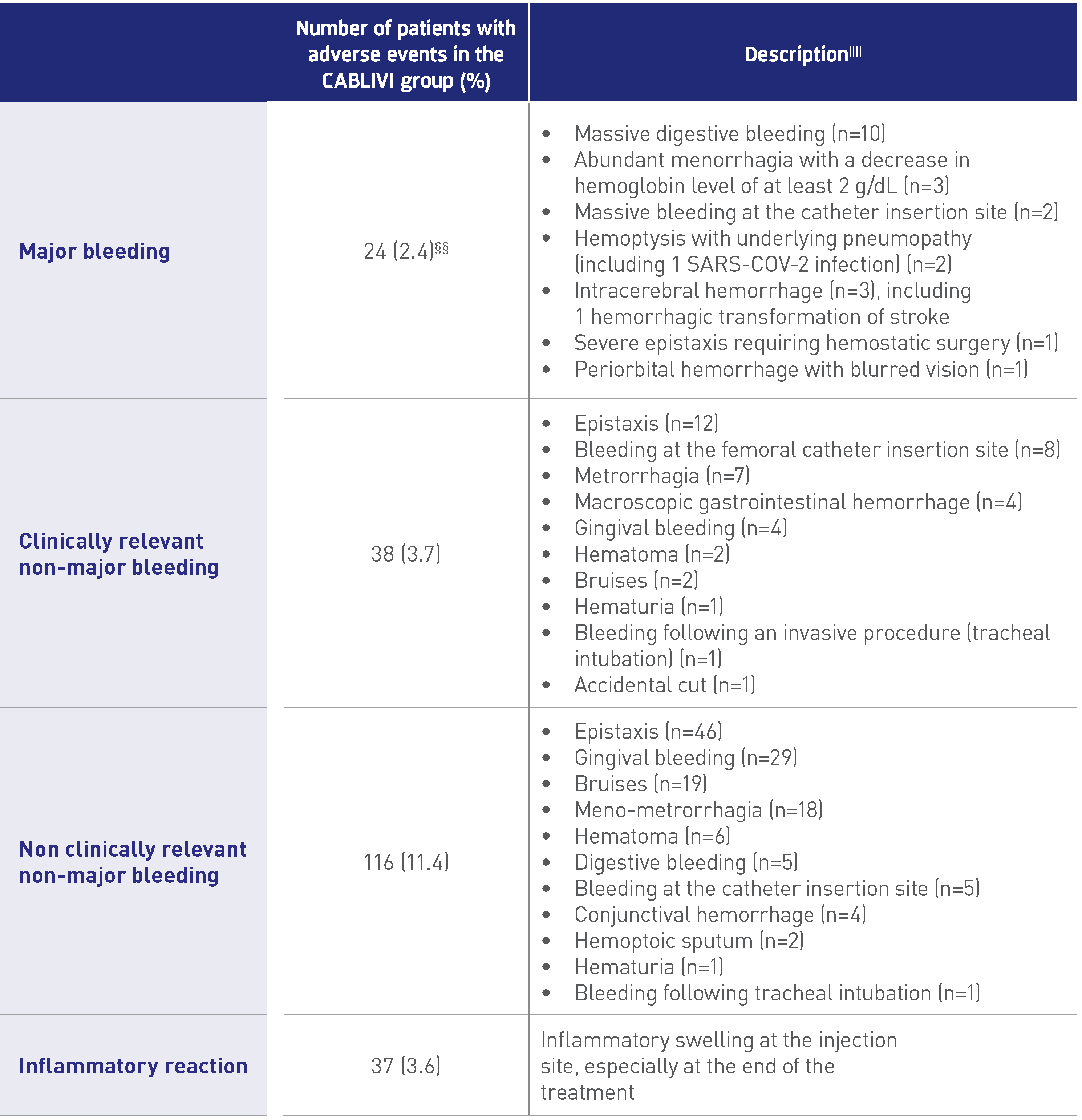.png)
§§Data are missing for 2 patients.
||||24 patients experienced 2 adverse events.
Hear what lead investigator Dr Paul Coppo has to say about the Capla 1000+ Project
Review the Capla 1000+ Project full publication
ADAMTS13=a disintegrin and metalloproteinase with a thrombospondin type 1 motif, 13; aTTP/iTTP=acquired/immune-mediated thrombotic thrombocytopenic purpura; HR=hazard ratio; LDH=lactate dehydrogenase; PEX=plasma exchange; TE=thromboembolic; TPE=therapeutic plasma exchange; ULN=upper limit of normal.
INDICATION
References: 1. Coppo P, Bubenheim M, Benhamou Y, et al. Caplacizumab use in immune-mediated thrombotic thrombocytopenic purpura: an international multicentre retrospective cohort study (the Capla 1000+ project). Lancet. 2025;82:1-13 and suppl. doi:10.1016/j.eclinm.2025.103168 2. CABLIVI. Prescribing information. Sanofi. 3. Scully M, Cataland SR, Peyvandi F, et al; HERCULES Investigators. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;380(4):335-346 and suppl/protocol. doi:10.1056/ NEJMoa1806311 4. Rituxan® (rituximab). Prescribing information. Genentech, Inc.
