Booster Dose: NUVAXOVID has a well established tolerability profile in patients aged 18+1,2
- ≤0.1% of patients experienced higher severity (Grade 4) reactions for each of the most frequent solicited adverse reactions1
- Patients can receive NUVAXOVID as their yearly updated COVID-19 shot, regardless of the previous COVID-19 vaccine brand they received1
Most frequent solicited adverse reactions after booster dose in adults aged 18-64 who received a NUVAXOVID primary series1
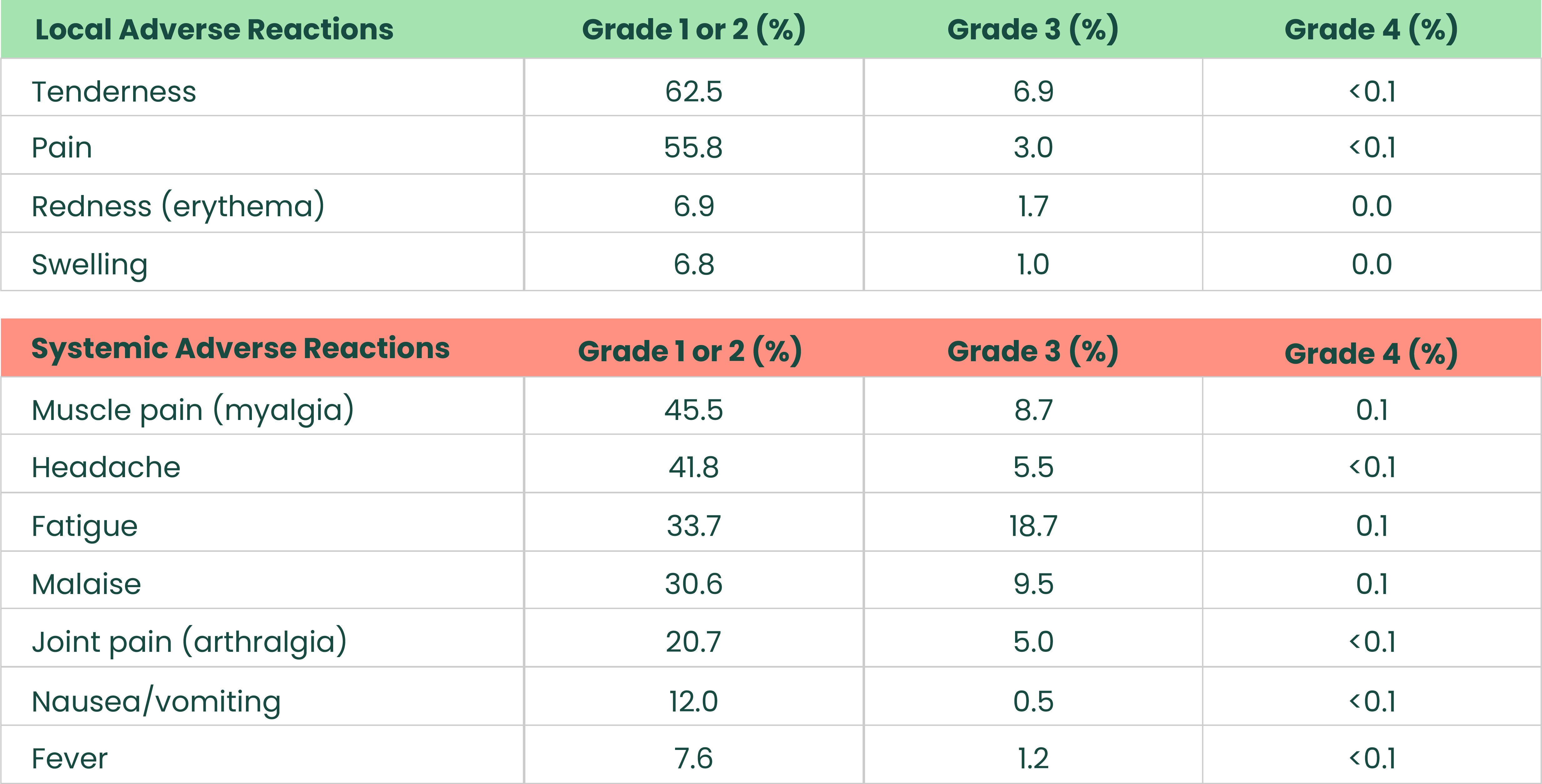.png)
Most frequent solicited adverse reactions after booster dose in adults aged 65+ who received a NUVAXOVID primary series1
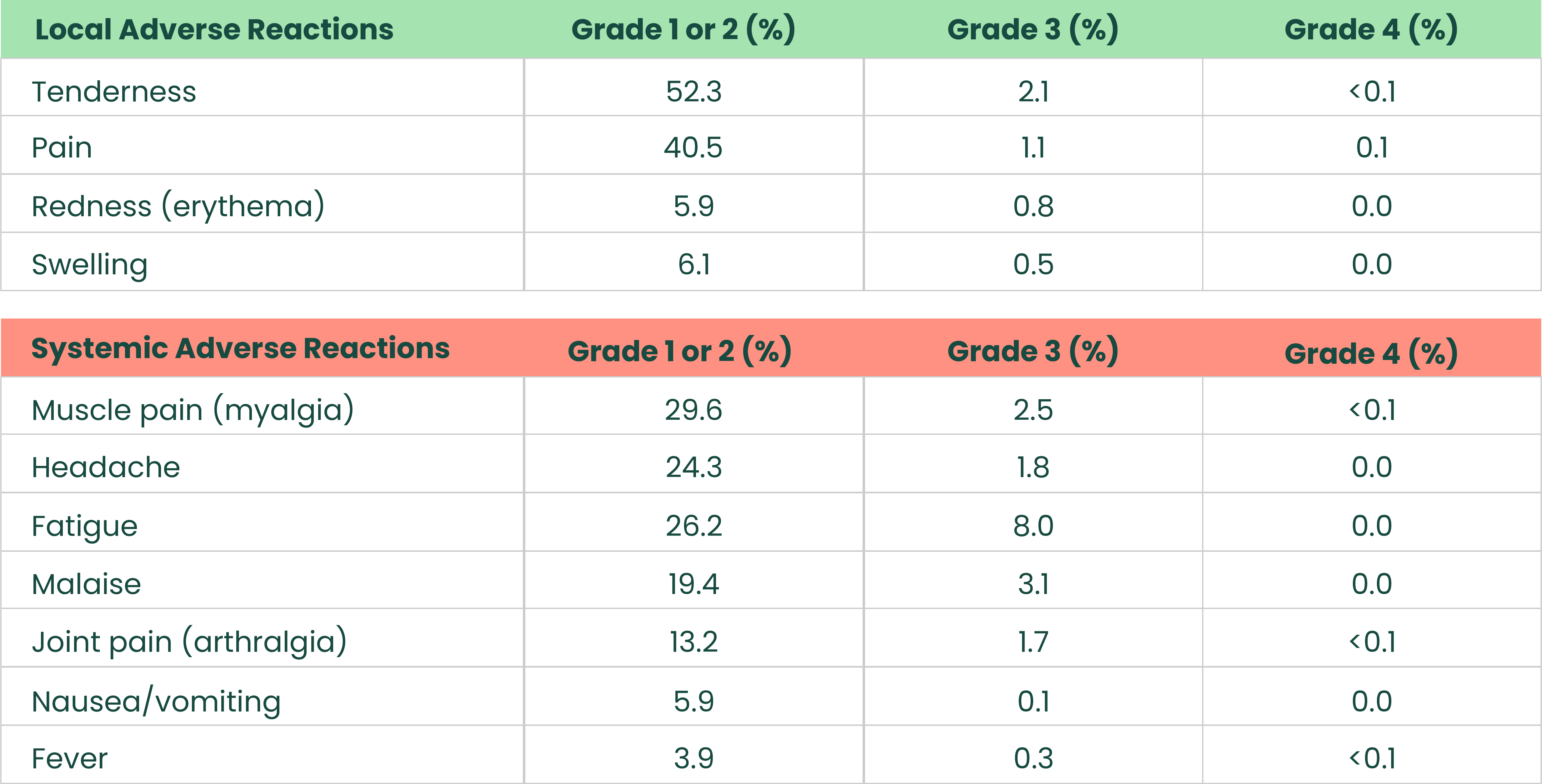.png)
The analysis included 9,817 participants 18 through 64 years and 1,630 participants ≥ 65 years who received the booster dose and completed at least one day of the vaccination reactogenicity diary, reported an unsolicited adverse event that was mapped to a reactogenicity term within 7 days of the dose, reported a symptom in the Daily Illness Symptoms Diary that was mapped to a reactogenicity term within 7 days of the dose, or reported a temperature in the Daily Illness Symptoms Diary within 7 days of the dose. Seven days included day of vaccination and the subsequent 6 days. Solicited reactogenicity events and use of antipyretic or pain medication were collected by the participant in the electronic diary (eDiary).1
2-Dose Primary Series: NUVAXOVID has a well established tolerability profile in patients aged 18-641,4
- With NUVAXOVID, ~84% of patients did not have their daily activities prevented by fatigue1
- For each of the other adverse reactions, ~94% of patients reported no higher than Grade 2 severity (see table below)1
- Less than 0.1% of patients experienced higher severity (Grade 4) reactions for each of the most frequent solicited adverse reactions1
Most frequent solicited adverse reactions after 2-dose primary series in adults aged 18-641
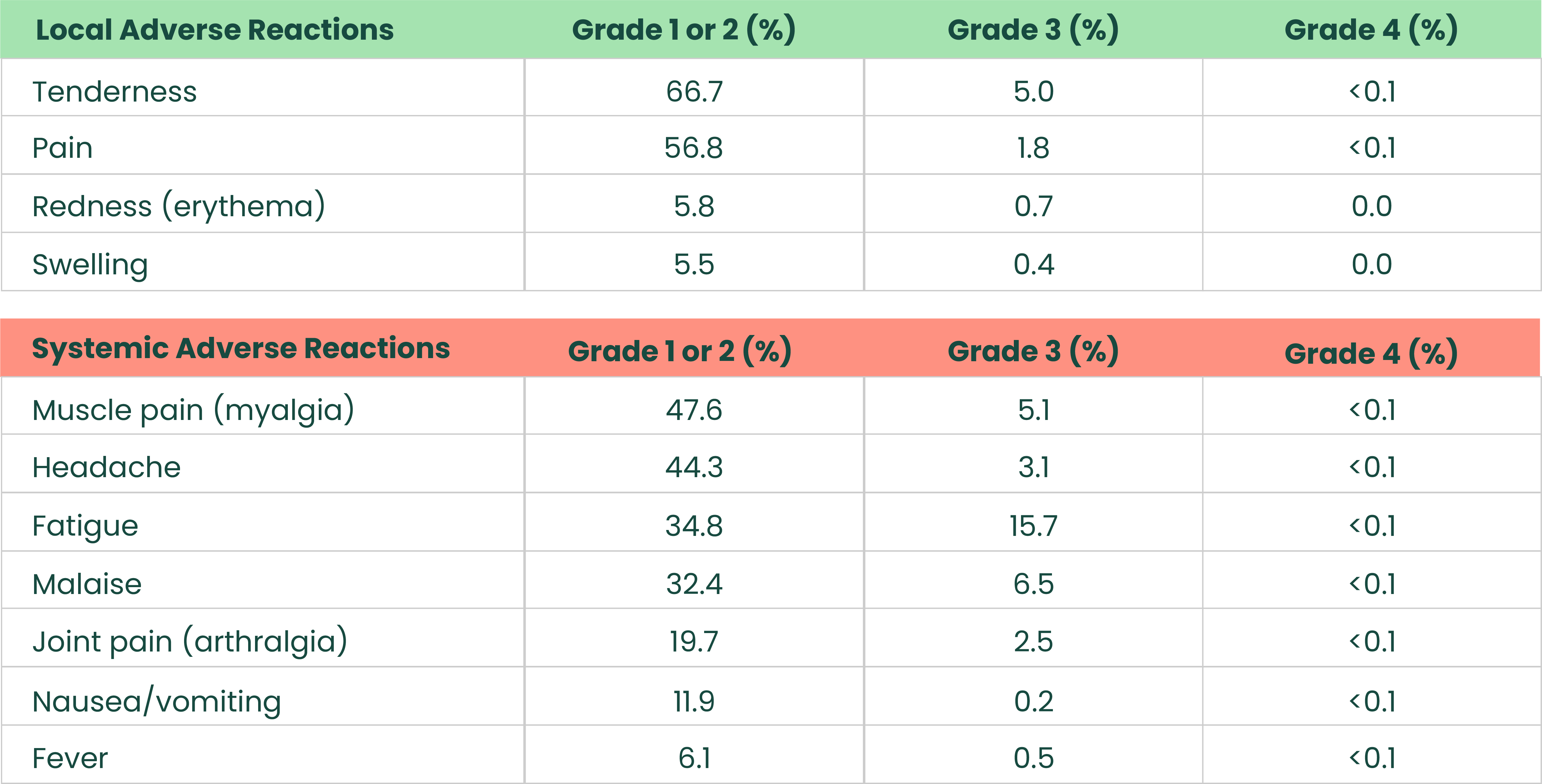.png)
The analysis included 16,041 (Dose 1) and 16,106 (Dose 2) participants in the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) group and 7,968 (Dose 1) and 7,859 (Dose 2) participants in the placebo group who received at least one dose and completed at least one day of the vaccination reactogenicity diary, reported an unsolicited adverse event that was mapped to a reactogenicity term within 7 days of the dose, reported a symptom in the Daily Illness Symptoms Diary that was mapped to a reactogenicity term within 7 days of the dose, or reported a temperature in the Daily Illness Symptoms Diary within 7 days of the dose. Seven days included day of vaccination and the subsequent 6 days. Solicited reactogenicity events and use of antipyretic or pain medication were collected by the participant in the electronic diary (eDiary).1
2-Dose Primary Series: NUVAXOVID has a well established tolerability profile in patients aged 65+1,2
- With NUVAXOVID, ~94% of patients did not have their daily activities prevented by fatigue1
- For each of the other adverse reactions, ~98% of patients reported no higher than Grade 2 severity (see table below)1
- Less than 0.1% of patients experienced higher severity (Grade 4) reactions for each of the most frequent solicited adverse reactions1
Most frequent solicited adverse reactions after 2-dose primary series in adults aged 65+1
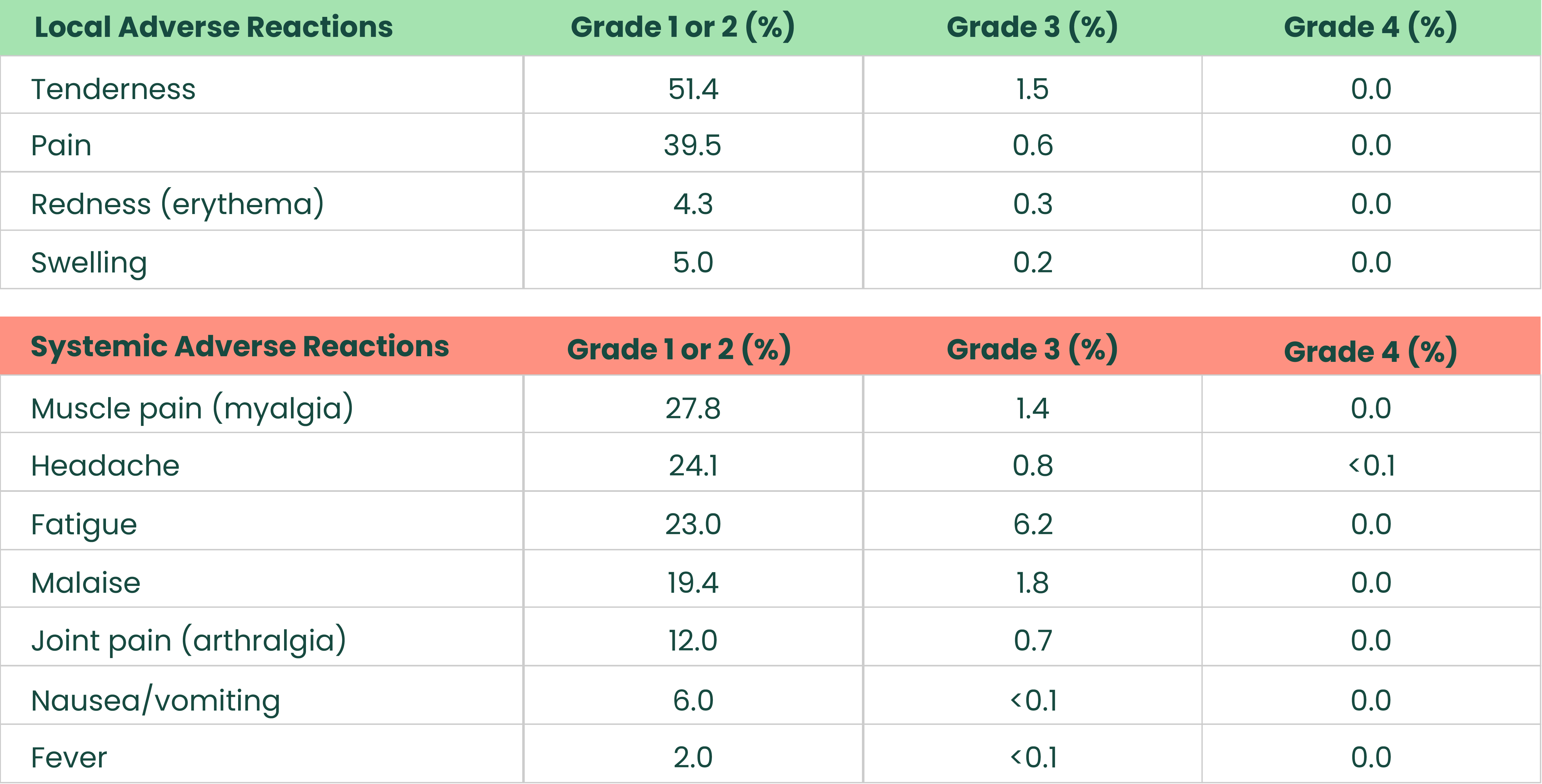.png)
The analysis included 2,293 (Dose 1) and 2,217 (Dose 2) participants in the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) group and 1,138 (Dose 1) and 1,057 (Dose 2) participants in the placebo group who received at least one dose and completed at least one day of the vaccination reactogenicity diary, reported an unsolicited adverse event that was mapped to a reactogenicity term within 7 days of the dose, reported a symptom in the Daily Illness Symptoms Diary that was mapped to a reactogenicity term within 7 days of the dose, or reported a temperature in the Daily Illness Symptoms Diary within 7 days of the dose. Seven days included day of vaccination and the subsequent 6 days. Solicited reactogenicity events and use of antipyretic or pain medication were collected by the participant in the electronic diary (eDiary).1
Unsolicited and serious adverse event rates were similar between NUVAXOVID and placebo groups1:
- The overall frequency of unsolicited adverse events in the pre-crossover period was similar in the NUVAXOVID group (11.8%) and the placebo group (11.0%)
- Serious adverse events were reported by 1.2% of participants in the NUVAXOVID group and 1.2% of participants in the placebo group
Grading scale definitions for adverse reactions1:
- Tenderness: Grade 1 = mild discomfort to touch; Grade 2 = discomfort with movement; Grade 3 = significant discomfort at rest; Grade 4 = emergency room (ER) visit or hospitalization
- Pain: Grade 1 = does not interfere with activity; Grade 2 = repeated use of non-narcotic pain reliever >24 hours or interferes with activity; Grade 3 = any use of narcotic pain reliever or prevents daily activity; Grade 4 = ER visit or hospitalization
- Redness (erythema): Grade 1 = 2.5–5 cm; Grade 2 = 5.1–10 cm; Grade 3 = >10 cm; Grade 4 = necrosis or exfoliative dermatitis
- Swelling: Grade 1 = 2.5–5 cm and does not interfere with activity; Grade 2 = 5.1–10 cm or interferes with activity; Grade 3 = >10 cm or prevents daily activity; Grade 4 = necrosis
- Fatigue, malaise, muscle pain, or joint pain (arthralgia): Grade 1 = no interference with activity; Grade 2 = some interference with activity; Grade 3 = significant; prevents daily activity; Grade 4 = ER visit or hospitalization
- Headache: Grade 1 = no interference with activity; Grade 2 = repeated use of non-narcotic pain reliever >24 hours or some interference with activity; Grade 3 = significant; any use of narcotic pain reliever or prevents daily activity; Grade 4 = ER visit or hospitalization
- Nausea/vomiting: Grade 1 = no interference with activity or 1–2 episodes/24 hours; Grade 2 = some interference with activity or >2 episodes/24 hours; Grade 3 = Prevents daily activity, requires outpatient IV hydration; Grade 4 = ER visit or hospitalization for hypotensive shock
- Fever: Grade 1 = 38.0–38.4°C (100.4–101.1°F); Grade 2 = 38.5–38.9°C (101.2–102.0°F); Grade 3 = 39.0–40°C (102.1–104°F); Grade 4 = >40°C (>104°F)
Recommend NUVAXOVID and offer your eligible patients a protein-based COVID-19 vaccine, with a manageable side-effect profile.1
INDICATION
References
1. NUVAXOVID. Prescribing Information. Novavax, Inc. 2. Dunkle LM, Kotloff KL, Gay CL, et al; 2019nCoV-301 Study Group. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. 2022;386(6):531-543. doi:10.1056/NEJMoa2116185
