- Artikel
- Bron: Campus Sanofi
- 10 jul 2025
Expert-presentatie: de ernstige gevolgen van een exacerbatie

My name is Surya Bhatt. I'm a professor of medicine at the University of Alabama at Birmingham, and I'm a COPD researcher. This talk is sponsored by Sanofi and Regeneron, and you won't get any CME credit. One of the other things that's very important in COPD is acute exacerbations. And these are not just an inconvenience but are associated with significant disease progression. Now there's plenty of data to suggest that these exacerbations are associated with lung function decline. They're commonly caused by infections such as viruses and bacterial infections sometimes due to environmental pollution and occupational exposures. Regardless of the cause, the severity is determined by where they're treated, which is not ideal. But if they manage to adjust steroids and antibiotics at home, that classifies as a moderate exacerbation. But if it's severe enough to end up in the hospital, and then that causes a severe exacerbation and people will suffer from exacerbations, have a faster rate of lung function decline than those who do not have exacerbations at all. And here on the right is data from the COPD gene study where they looked at the FEV one decline by exacerbation or the frequency of exacerbations.
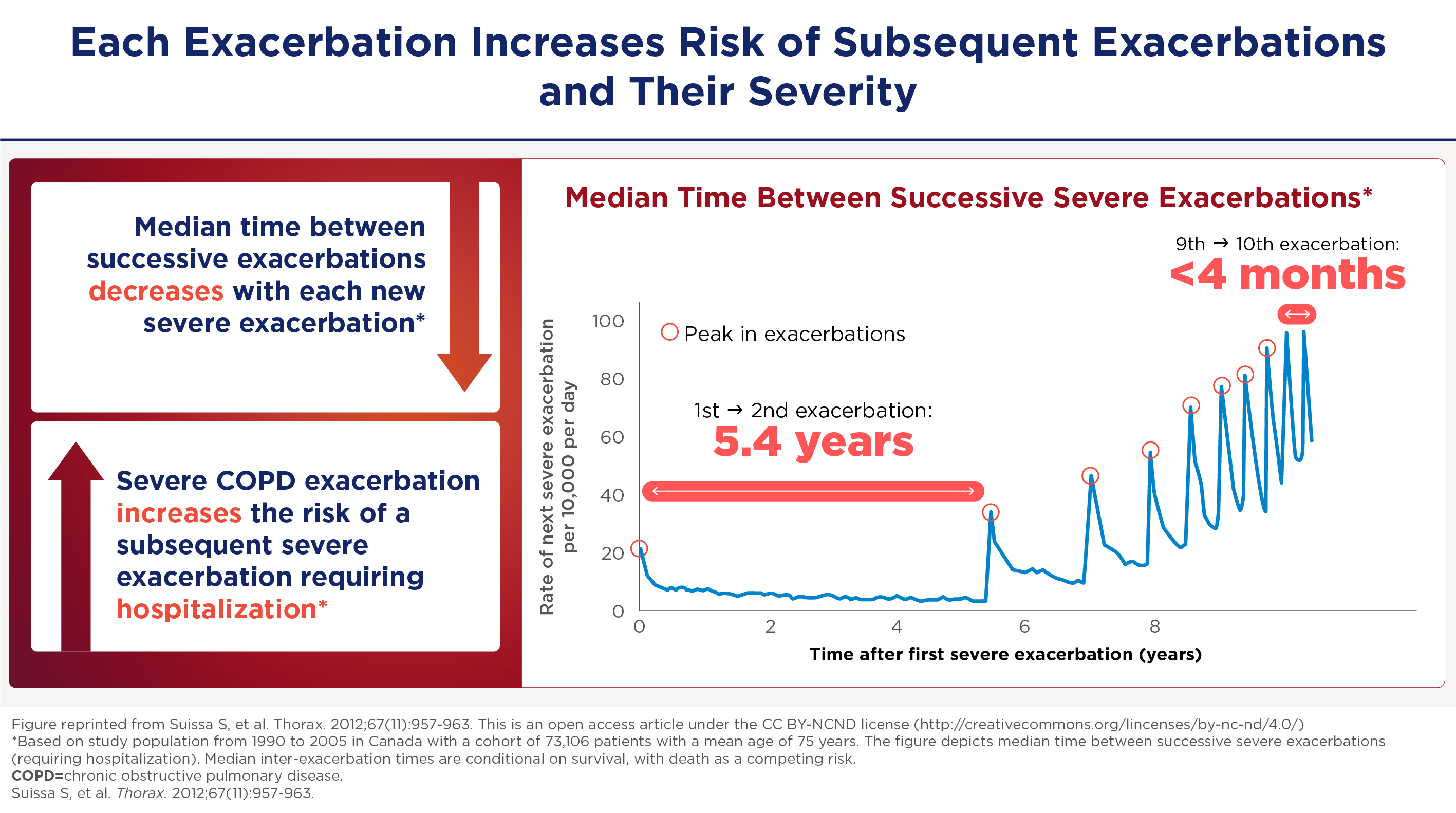
And especially for severe exacerbations and especially for those with milder disease who have more lung function to lose the effect can be quite dramatic. The FEV even decline can be up to 87mm for a severe exacerbation in world stage one, for example. And as you can imagine, if you get 4 or 5 exacerbations, you can lose up to a half a meter of fev1 over the next 4 to 5 years. The severe exacerbations appear to be sentinel events in the course of the COPD patients. When somebody gets a first exacerbation it seems like it begets more exacerbations. In a retrospective study by Sammy Sosa of about 75,000 patients in Canada. He modeled the severe exacerbation frequency and found that the usual median interval between a first and a second exacerbation is about five and a half years, and then the exacerbations happen at progressively shorter intervals to the point that by about the 10th exacerbation the median interval is about four months. So you can imagine a patient within a few years settling into a pattern of 3 to 4 exacerbations needing hospitalization in a single year.
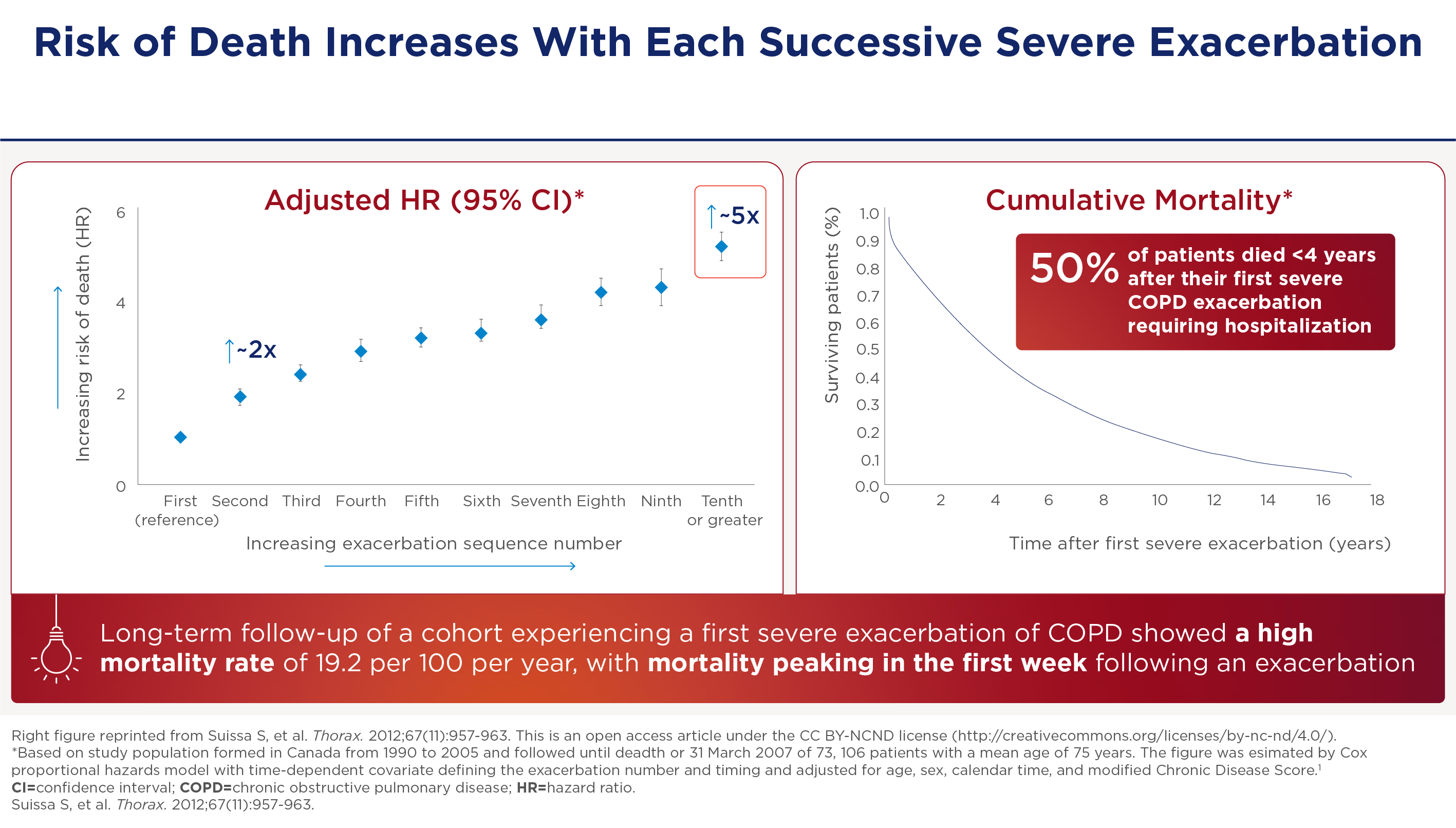
And this is not without consequence. These kind of exacerbations can have an effect on mortality. The hazards of mortality is about twice. If you get two severe exacerbations as opposed to one severe exacerbation. And the hazards of mortality is five fold. If you get ten exacerbations as opposed to one severe exacerbation. So there's almost a monotonic increase in the hazards with increasing severe exacerbation frequency. And the curve on the right shows your risk of dying after a single severe exacerbation requiring hospitalization. So the mortality within one year is about 25%, and the mortality within four years after discharge after the first severe exacerbation is 50%.
De slopende rol van exacerbaties bij de progressie van COPD
Acute exacerbaties zijn zowel hinderlijke als slopende gebeurtenissen, die leiden tot significante ziekteprogressie met achteruitgang van de longfunctie. Deze achteruitgang is ernstiger bij frequente exacerbaties. Zo laat de Gene-studie een correlatie zien tussen de exacerbatiefrequentie en de snelheid van FEV1-afname.
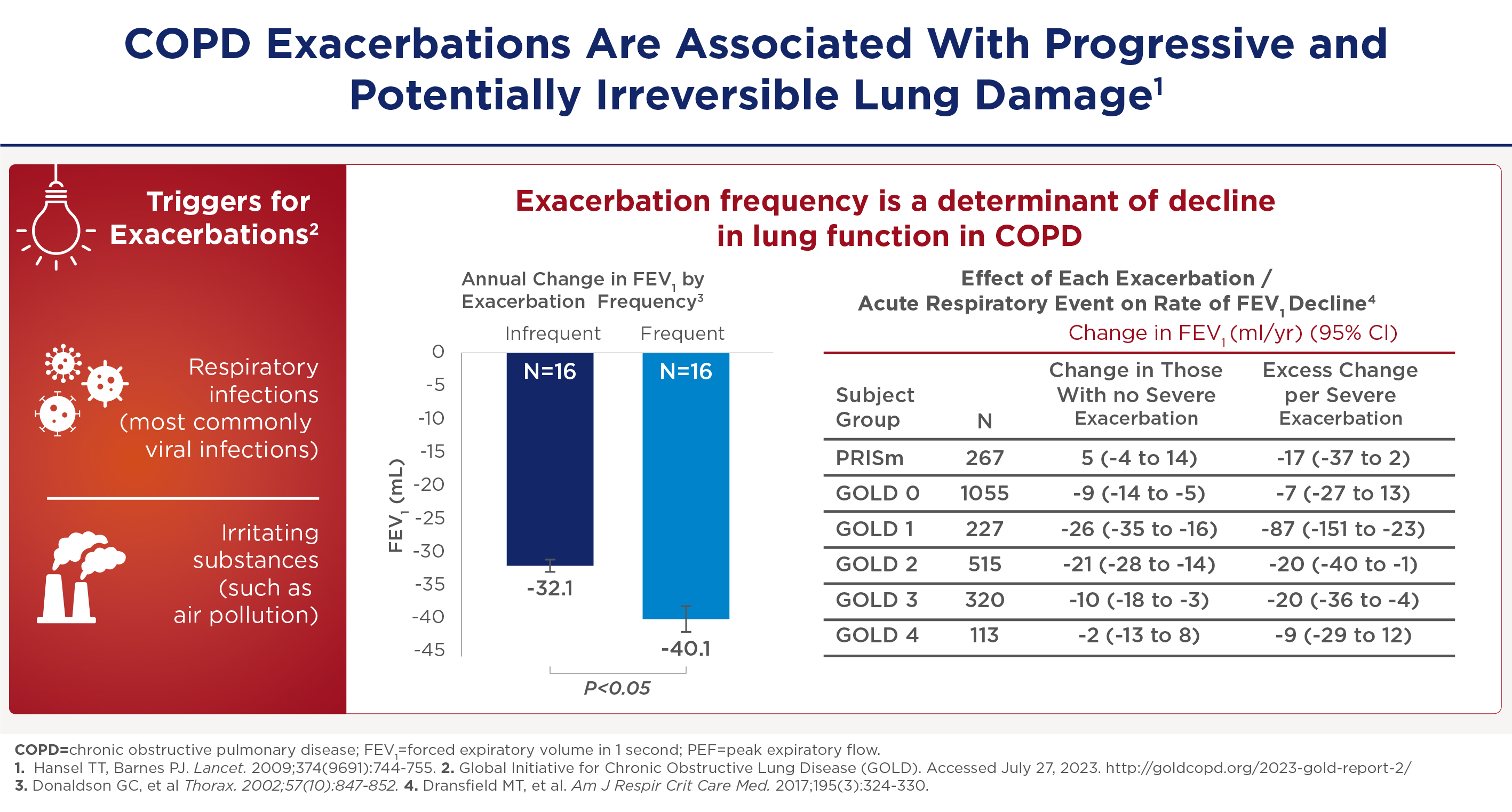
Frequente exacerbaties -zowel matige als ernstige- verminderen de longfunctie.
Opeenvolgende exacerbaties brengen extra risico
Ernstige exacerbaties zijn bijzonder zorgwekkend vanwege hun cascade-effecten. In de studie van Sammy Suissa et al -waarin de frequentie van ernstige exacerbaties werd gemodelleerd bij ongeveer 75.000 patiënten- bleek dat het exacerbatie-interval steeds korter werd. Ook dit wijst op ziekteprogressie met achteruitgang van de longfunctie:
Meer exacerbaties geven hogere mortaliteit
De sterfterisico's die gepaard gaan met ernstige exacerbaties zijn aanzienlijk: de kans op sterfte is ongeveer twee keer zo groot als je twee ernstige exacerbaties krijgt in vergelijking met één ernstige exacerbatie. En de kans op sterfte is vijf keer zo groot als je tien exacerbaties krijgt in vergelijking met één ernstige exacerbatie. Bovendien is het risico om binnen een jaar te overlijden na een ernstige exacerbatie ongeveer 25%. Dit loopt op tot 50% binnen vier jaar na ontslag uit het ziekenhuis.
* De referenties en de bovenstaande inhoud zijn gebaseerd op de presentatie van Dr. Bhatt; zie de video voor meer informatie.
Samenvatting
Professor Surya Bhatt belicht de slopende, progressieve gevolgen van COPD-exacerbaties. Daarom blijven preventieve strategieën belangrijke onderdelen van de behandeling. Tegelijkertijd geven nieuwe inzichten in de triggers en inflammatie-mechanismen ook nieuwe aangrijpingspunten van de behandeling.
-
Gandhi NA, Bennett BL, Graham NMH, et al. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15(1):35-50.
-
Yousuf A, Ibrahim W, Greening NJ, et al. T2 Biologics for Chronic Obstructive Pulmonary Disease. J Allergy Clin Immunol Pract. 2019;7(5):1405-1416.
-
Aghapour M, Raee P, Moghaddam SJ, et al. Airway Epithelial Barrier Dysfunction in Chronic Obstructive Pulmonary Disease: Role of Cigarette Smoke Exposure. Am J Respir Cell Mol Biol. 2018;58(2):157-169.
-
Barnes JP. Inflammatory endotypes in COPD. Allergy. 2019;74(7):1249-1256.
-
Wang Z, Bafadhel M, Haldar K, et al. Lung microbiome dynamics in COPD exacerbations. Eur Respir J. 2016;47(4):1082-1092.
-
Smithgall MD, Comeau MR, Yoon BRP, et al. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20(8):1019-1030.
-
Senra L, Mylonas A, Kavanagh RD, et al. IL-17E (IL-25) enhances innate immune responses during skin inflammation. J Invest Dermatol. 2019;139(8):1732-1742.
-
Linden D, Guo-Parke H, Coyle PV, et al. Respiratory viral infection: a potential “missing link” in the pathogenesis of COPD. Eur Respir Rev. 2019; 28(151):180063.
-
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Accessed July 27, 2023. https://goldcopd.org/2023-gold-report-2/.
-
Calderon AA, Dimond C, Choy DF, et al. Targeting interleukin-33 and thymic stromal lymphopoietin pathways for novel pulmonary therapeutics in asthma and COPD. Eur Respir Rev. 2023;32(167):220144.
-
Kurokawa M, Matsukura S, Kawaguchi M, et al. Interleukin-33-activated dendritic cells induce the production of thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Arch Allergy Immunol. 2013;161(Suppl 2):52-57.
-
Griesenauer B, Paczesny S. The ST2/IL-33 axis in immune cells during inflammatory diseases. Front Immunol. 2017;8:475.
-
Borowczyk J, Shutova M, Brembilla NC, et al. IL-25 (IL-17E) in epithelial immunology and pathophysiology. J Allergy Clin Immunol. 2021;148(1):40-52.
-
Claudio E, Wang H, Kamenyeva O, et al. IL-25 orchestrates activation of Th cells via conventional dendritic cells in tissue to exacerbate chronic house dust mite–induced asthma pathology. J Immunol. 2019;203(8)2319-2327.
-
Kotlyarov S. Involvement of the innate immune system in the pathogenesis of chronic obstructive pulmonary disease. Int J Mol Sci. 2022;23(2):985.
-
Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425-437.
-
Saatian B, Rezaee F, Desando S, et al. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers. 2013;1(2):e24333.
-
Zheng T, Zhu Z, Wang Z, et al. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest. 2000;106(9):1081-1093.
-
Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103(6):779-788.
-
Alevy YG, Patel AC, Romero AG, et al. IL-13-induced airway mucus production is attenuated by MAPK13 inhibition. J Clin Invest. 2012;122(12):4555-4568.
-
Wang X, Xu C, Ji J, et al. IL-4/IL-13 upregulates Sonic hedgehog expression to induce allergic airway epithelial remodeling. Am J Physiol Lung Cell Mol Physiol. 2020;318(5):L888-L899.
-
Cooper PR, Poll CT, Barnes PJ, et al. Involvement of IL-13 in tobacco smoke-induced changes in the structure and function of rat intrapulmonary airways. Am J Respir Cell Mol Biol. 2010;43(2):220-226.
-
Doyle AD, Mukherjee M, LeSuer WE, et al. Eosinophil-derived IL-13 promotes emphysema. Eur Respir J. 2019;53(5):1801291.
-
Sun J, Liu T, Yan Y, et al. The role of Th1/Th2 cytokines played in regulation of specific CD4+ Th1 cell conversion and activation during inflammatory reaction of chronic obstructive pulmonary disease. Scand J Immunol. 2018;88(1):e12674.
-
Kim CH, Kim KE, Yoon JH, et al. Upregulation of MUC5AC gene expression by IL-4 through CREB in Human airway epithelial cells. J Cell Biochem. 2009;108(4):974-981.
-
Yu H, Li Q, Kolosov VP, et al. Interleukin-13 induces mucin 5AC production involving STAT6/SPDEF in human airway epithelial cells. Cell Commun Adhes. 2010;17(4-6):83-92.
Neem contact op

MAT-NL-2500431