When every bleed counts, ALPROLIX can help protect* pediatric patients every step of the way
*ALPROLIX has been proven to help patients prevent bleeding episodes using a prophylaxis regimen.1
ALPROLIX® was studied in more pediatric PTPs than any other factor IX EHL
In PTPs ≤ 11 years
MEDIAN OVERALL ABR

KIDS B-LONG TRIAL
(IP PATIENTS)
(0-3.1)

KIDS B-LONG TRIAL
(IP PATIENTS)
(0-3.1)

MEDIAN AsBR
(IP PATIENTS)
(0-1.2)

MEDIAN JOINT ABR
(IP PATIENTS)
(0-1.1)

BLEEDS FOR 1 IN 3 PATIENTS
(IP PATIENTS)
(n=10/30)
Pediatric patients were studied up to 4.8 years, from the beginning of the kinds B-Long trial to the end of the B-Yond Trial2,11
†From the individualized prophylaxis arm of the Kids B-LONG trial.[RM(F1]
‡10 out of 30 children, or 33%, reported no bleeds at all during the Kids B-LONG trial.
ALPROLIX was demonstrated effective in the B-LONG trial, and long-term efficacy in the B-YOND trial
Kids B-LONG was a phase 3 open-label study investigating the safety and efficacy of ALPROLIX in 30 PTPs aged ≤11 years with severe hemophilia. The number of patients 1 to 5 years of age was 15, and 6 to 11 years of age was 15. All 30 patients were treated with ALPROLIX on an individualized prophylactic regimen.
B-YOND was an open-label extension trial that studied the long-term safety and efficacy of ALPROLIX over 5 years in 120 adult, adolescent, and pediatric patients previously treated in Kids B-LONG or B-LONG. Study arms included: fixed-interval (n=74), fixed-dose (n=36), modified prophylaxis (n=17), and on-demand (n=15).x
column1
option 2
test tets
ALPROLIX can help protect* PUPs patients for the journey ahead
In PUPs ≤2 years

MEDIAN OVERALL ABR
(PUPs B-LONG TRIAL)†
(0-2.5)

MEDIAN AsBR
(PROPHYLAXIS PATIENTS)
(0-0)

MEDIAN TRAUMATIC JOINT ABR
(PROPHILAXIS PATIENTS)
(0-0)

MEDIAN SPONTANEOUS JOINT ABR
(PROPHYLAXIS PATIENTS)
(0-0)
From the prophylaxis arm of the PUPs B-LONG trial.
ALPROLIX was demonstrated effective in the PUPs B-LONG trial
PUPs B-LONG was a phase 3 open-label study investigating the safety and efficacy of ALPROLIX in 33 PUPs aged <18 years with moderate or severe hemophilia B. At enrollment, the median age was 0.6 years (0.1-2 years). Study arms included: prophylaxis (n=28) and on-demand (n=5).
ALPROLIX® was studied in more pediatric PTPs than any other factor IX EHL
In PTPs ≤ 11 years
MEDIAN OVERALL ABR

KIDS B-LONG TRIAL
(IP PATIENTS)
(0-3.1)

KIDS B-LONG TRIAL
(IP PATIENTS)
(0-3.1)

MEDIAN AsBR
(IP PATIENTS)
(0-1.2)

MEDIAN JOINT ABR
(IP PATIENTS)
(0-1.1)

BLEEDS FOR 1 IN 3 PATIENTS
(IP PATIENTS)
(n=10/30)
Pediatric patients were studied up to 4.8 years, from the beginning of the kinds B-Long trial to the end of the B-Yond Trial2,11
†From the individualized prophylaxis arm of the Kids B-LONG trial.[RM(F1]
‡10 out of 30 children, or 33%, reported no bleeds at all during the Kids B-LONG trial.
ALPROLIX was demonstrated effective in the B-LONG trial, and long-term efficacy in the B-YOND trial
Kids B-LONG was a phase 3 open-label study investigating the safety and efficacy of ALPROLIX in 30 PTPs aged ≤11 years with severe hemophilia. The number of patients 1 to 5 years of age was 15, and 6 to 11 years of age was 15. All 30 patients were treated with ALPROLIX on an individualized prophylactic regimen.
B-YOND was an open-label extension trial that studied the long-term safety and efficacy of ALPROLIX over 5 years in 120 adult, adolescent, and pediatric patients previously treated in Kids B-LONG or B-LONG. Study arms included: fixed-interval (n=74), fixed-dose (n=36), modified prophylaxis (n=17), and on-demand (n=15).x
column1
option 2
test tets
ALPROLIX can help protect* PUPs patients for the journey ahead
In PUPs ≤2 years

MEDIAN OVERALL ABR
(PUPs B-LONG TRIAL)†
(0-2.5)

MEDIAN AsBR
(PROPHYLAXIS PATIENTS)
(0-0)

MEDIAN TRAUMATIC JOINT ABR
(PROPHILAXIS PATIENTS)
(0-0)

MEDIAN SPONTANEOUS JOINT ABR
(PROPHYLAXIS PATIENTS)
(0-0)
From the prophylaxis arm of the PUPs B-LONG trial.
ALPROLIX was demonstrated effective in the PUPs B-LONG trial
PUPs B-LONG was a phase 3 open-label study investigating the safety and efficacy of ALPROLIX in 33 PUPs aged <18 years with moderate or severe hemophilia B. At enrollment, the median age was 0.6 years (0.1-2 years). Study arms included: prophylaxis (n=28) and on-demand (n=5).
Dosing simplicity for pediatric patients and their caregivers
One single, simple starting dose: For children aged ≤11 years, the recommended prophylaxis starting regimen is 60 IU/kg once weekly
Flexibility for the unexpected: Dose and regimen can be adjusted based on patient response
~93% of pediatric patients maintained or extended their dosing interval through the B-YOND trial.
ALPROLIX on-demand: bleed control for pediatric PTPs and PUPs
ALPROLIX stopped most bleeds with 1 or 2 infusions
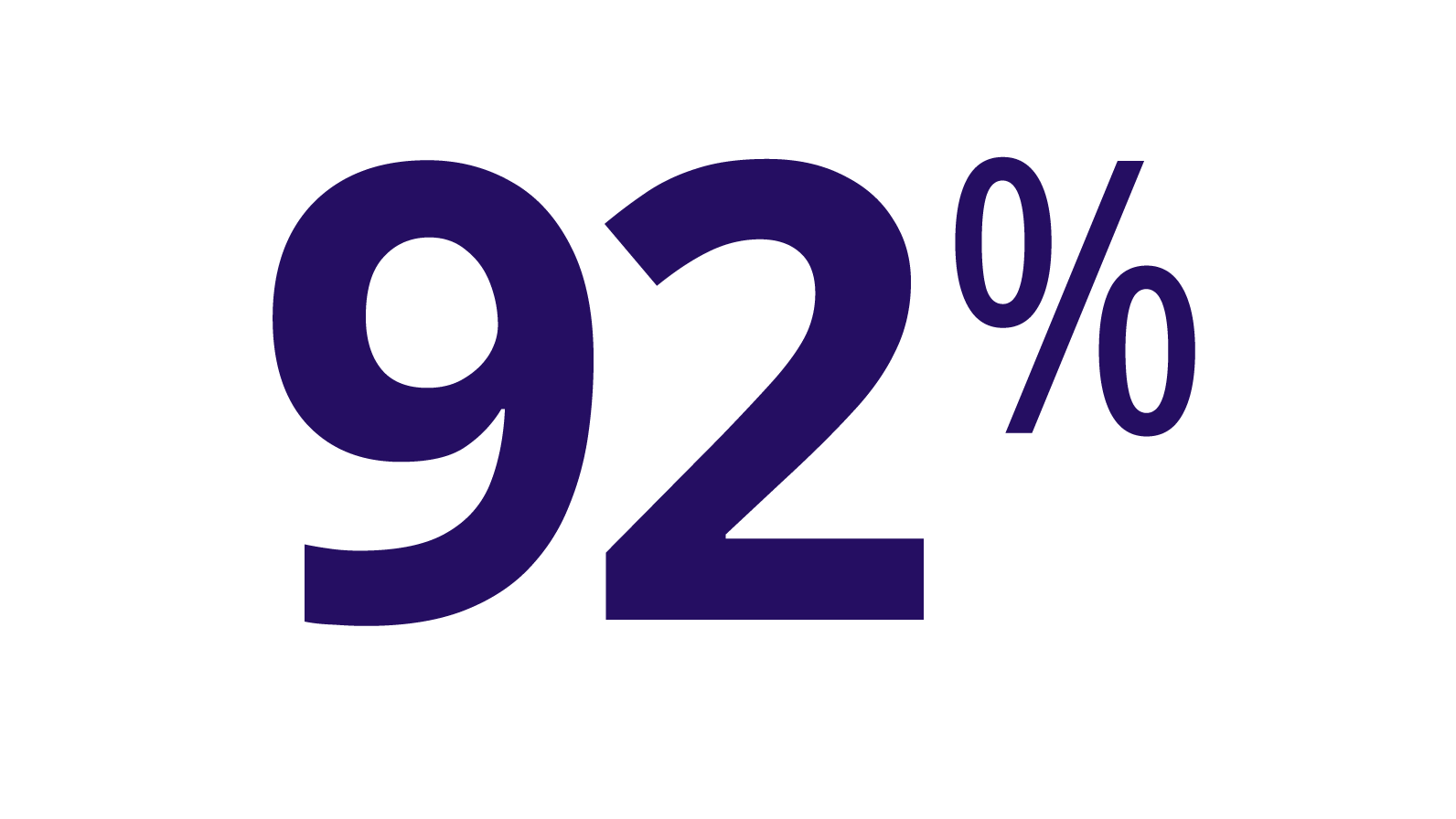
KIDS B-LONG TRIAL
(n=55/60)
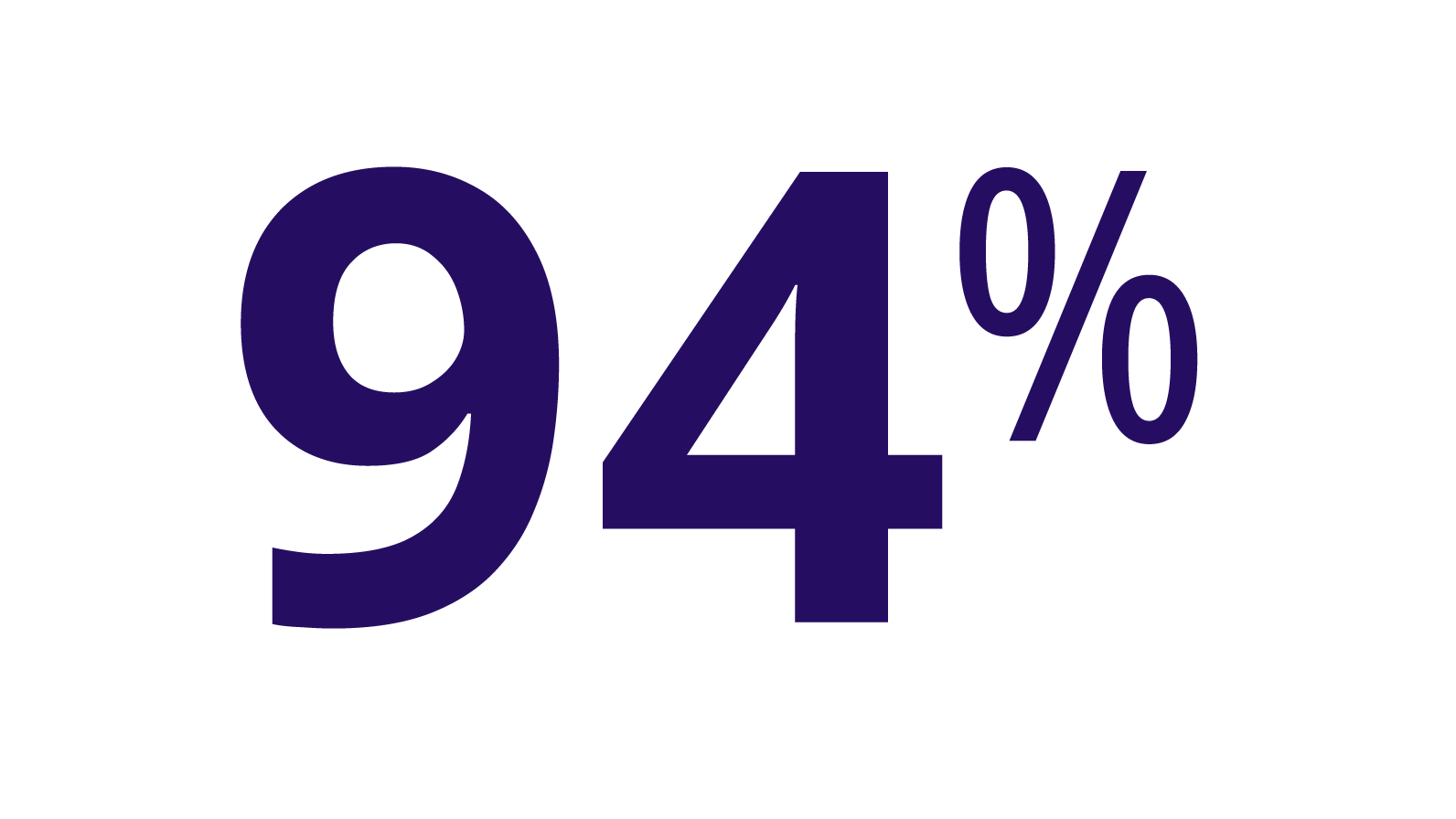
PUPs B-LONG TRIAL
(n=80/85)
Response to first infusion was rated good or excellent*

KIDS B-LONG TRIAL
(n=47/53)

PUPs B-LONG TRIAL
(n=72/79)
During the trials, 7 first injections in children were not evaluated for response, so they are not included in this analysis.
Excellent response was defined as sudden pain relief and/or improvement in signs of bleeding. Good response was defined as definite pain relief and/or improvement in signs of bleeding but possibly requiring another injection in 1 or 2 days.

ALPROLIX offers pediatric patients hemostatic control for both major and minor surgeries
Spotlight on pediatric surgical patients
While the ALPROLIX surgical data was pooled, here is how the pediatric subpopulation is reflected in these numbers:
- Major surgeries in pediatric PTPs: 1 (B-YOND Trial)
- Minor surgeries in pediatric PTPs: 3 (B-LONG Trial) + 2 (B-YOND Trial)
Perioperative efficacy across all ages

|
35 MAJOR SURGERIES22 |
62 MINOR SURGERIES1 | ||
|
Joint replacement/revision (n=10) |
Tooth extraction (n=24) | ||
|
Abdominal (n=6) |
Eye surgery (n=5) | ||
|
Other orthopedic (n=5) |
Oral surgery (n=5) | ||
|
Fracture and fixation (n=3) |
Incision and drainage (n=5) | ||
|
Arthroscopy (n=2) |
Vascular procedure (n=5) | ||
|
Spinal surgery (n=2) |
Minor orthopedic (n=4) | ||
|
Cranial/brain (n=2) |
Other non-orthopedic (n=4) | ||
|
Other non-orthopedic (n=2) |
Other dental (n=4) | ||
|
Joint fusion (n=2) |
Port placement or removal (n=3) | ||
|
Dental (n=1) |
Minor skin procedure (n=2) | ||
|
|
Endoscopy with/without procedure (n=1) | ||
|
22 Patients |
37 patients |
*Patients were classified by parent study. Data were derived from surgeries performed during the B-LONG trial, Kids B-LONG trial, and B-YOND extension trial. Those who underwent major and minor surgery were included in both cohorts.8 Subjects had more than one major surgery.
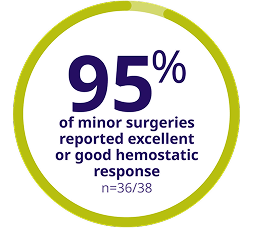
Patients undergoing major surgery had an excellent or good hemostatic response with ALPROLIX
- The median average dose per injection to maintain hemostasis during surgery was 94.7 IU/kg (range: 49 to 152). Perioperative factor IX replacement with ALPROLIX was by bolus infusion only. The safety of continuous infusion was not evaluated
- 80% (28 of 35) of the major surgeries required a single perioperative dose to maintain hemostasis during surgery
- Hemostasis was assessed by the investigator after surgery

MAJOR SURGERIES: B-LONG, KIDS. B-LONG, AND B-YOND TRIALS
.png)
MAJOR SURGERIES: B-LONG, KIDS. B-LONG, AND B-YOND TRIALS
*Out of 35 major surgeries, 33 were assessed in 22 subjects.
†8 subjects had more than 1 major surgery.
‡Out of 62 minor surgeries, 38 were assessed in 37 subjects.

ALPROLIX clinical results are consistent with real-world observations
ABR=annualized bleed rate; AsBR=annualized spontaneous bleed rate; PTP=previously-treated patient.
INDICATION:
References: 1. ALPROLIX [package insert]. Waltham, MA: Bioverativ Therapeutics Inc. 2. Fischer K, Kulkarni R, Nolan B, et al. Recombinant factor IX Fc fusion protein in children with haemophilia B (Kids B-LONG): results from a multicentre, non-randomised phase 3 study. Lancet Haematol. 2017;4(2):e75-e82. 3. Powell JS, Pasi KJ, Ragni MV, et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369(24):2313-2323. 4. Data on file. Waltham, MA; Bioverativ Therapeutics Inc. 5. Blanchette VS, Key NS, Ljung LR, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935-1939. 6. Pasi KJ, Fischer K, Ragni M, et al. Long-term safety and sustained efficacy for up to 5 years of treatment with recombinant factor IX Fc fusion protein in subjects with haemophilia B: results from the B-YOND extension study. Haemophilia. 2020;26(6):e262-e271. 7. Nolan B, Klukowska A, Shapiro A, et al. Final results of PUPs B-LONG study: evaluating safety and efficacy of rFIXFc in previously untreated patients with hemophilia B. Poster presented at: The 28th Congress of the International Society on Thrombosis and Haemostasis (ISTH); July 12-14, 2020; Virtual.