ALPROLIX is the only factor IX product to utilize half-life extension with Fc Fusion technology1-3
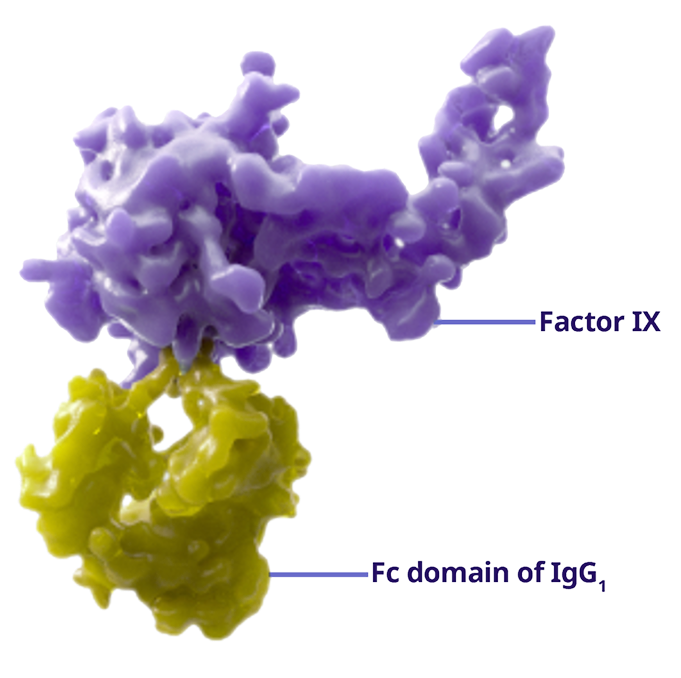
- ALPROLIX is composed of rFIX covalently linked to the Fc region of IgG11,4
- IgG1 is a naturally occurring protein with anti-inflammatory properties1,4
- The natural recycling pathway of IgG1 allows ALPROLIX to stay in the body for an extended period of time, delaying degradation and extending half life1
- ALPROLIX does not accumulate in the body5

ALPROLIX is the only EHL to mimic the pathway of natural factor IX, going to where it is needed2,6
ALPROLIX mirrors the pathway of natural FIX by leaving the bloodstream and distributing in the extravascular space1,2
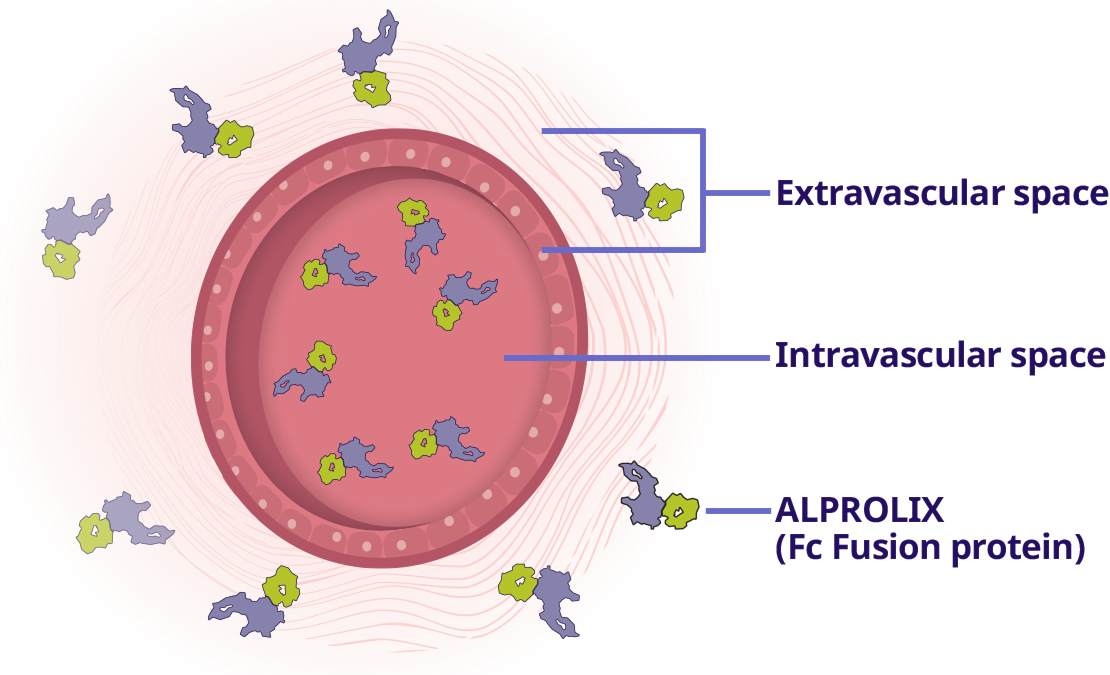
ALPROLIX uses Fc Fusion to extend the period of time it remains in the body vs BeneFIX®1,6

ALPROLIX reaches peak activity in 10 minutes—as quickly as BeneFIX [coagulation factor IX (recombinant)]6*†

ALPROLIX stays in circulation more than twice as long as BeneFIX6

The half life of ALPROLIX was 82 hours vs 34 hours for BeneFIX6
STUDIES HAVE NOT BEEN CONDUCTED TO ASSESS THE SAFETY OR EFFICACY OF ALPROLIX COMPARED WITH BENEFIX.
* A subset of 22 patients (the sequential pharmacokinetic subgroup) received consecutive single IV doses of 50 IU/kg BeneFIX and ALPROLIX at the beginning of the B-LONG study (baseline) for direct comparison. For both ALPROLIX and BeneFIX, peak activity was reached approximately 10 minutes after the start of the infusion.6,7
† Peak activity level does not mean bleeds are resolved within 10 minutes.6
EHL=extended half-life; FIX=factor IX; IgG1=immunoglobulin G1; rFIX=recombinant factor IX.
Experience Fc Fusion technology in action
INDICATION:
References: 1. ALPROLIX. Package insert. Bioverativ Therapeutics Inc; 2023. 2. Iorio A, Fischer K, Blanchette V, Rangarajan S, Young G, Morfini M; Pharmacokinetic (PK) Expert Working Group of the International Prophylaxis Study Group (the IPSG). Tailoring treatment of haemophilia B: accounting for the distribution and clearance of standard and extended half-life FIX concentrates. Thromb Haemost. 2017;117(6):1023-1030. 3. Diao L, Li S, Ludden T, Gobburu J, Nestorov I, Jiang H. Population pharmacokinetic modelling of recombinant factor IX Fc fusion protein (rFIXFc) in patients with haemophilia B. Clin Pharmacokinet. 2014;53(5):467-477. 4. Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313(5787):670-673. 5. Shapiro A. Development of long-acting recombinant FVIII and FIX Fc fusion proteins for the management of hemophilia. Expert Opin Biol Ther. 2013;13(9):1287-1297. 6. Data on file. Waltham, MA; Bioverativ Therapeutics Inc. 7. Powell JS, Pasi KJ, Ragni MV, et al; B-LONG Investigators. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369(24):2313-2323.

