Factors VIII and IX distribute differently in the body1-3
Factor VIII (missing in hemophilia A) is largely limited to the intravascular space (within the plasma)1-3
Factor IX (missing in hemophilia B) distributes to the extravascular space (tissues, muscles, and joints)3,4

Factor VIII binds to von Willebrand,
a protein located inside the plasma1-3

Factor IX binds to type IV collagen,
which is located outside the plasma5
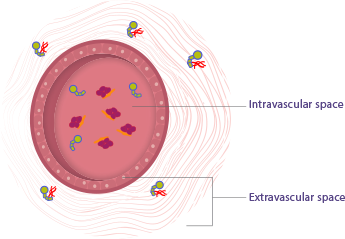
In the extravascular space, factor IX binds to type IV collagen and may play a role in coagulation, as shown in preclinical data. In hemophilia B, factor IX distributes outside of the plasma and into areas such as the tissues, muscles, and joints. Trough levels do not account for this extravascular distribution of infused factor IX.4,5
Notice: Conclusions regarding efficacy and safety in humans cannot be made based on results from preclinical studies.
It is important to evaluate factor IX replacement therapy based on outcomes such as bleed prevention5
Because of the limitations in pharmacokinetic evaluation for patients with hemophilia B, it is important to evaluate factor IX replacement therapy based on outcomes such as bleed prevention.
INDICATION:
References: 1. Lenting PJ, Schooten CJM van, Denis CV. Clearance mechanisms of von Willebrand factor and factor VIII. J Thromb Haemost. 2007;5(7):1353-1360. 2. Morfini M. The history of clotting factor concentrates pharmacokinetics. J Clin Med. 2017;6(3):35. 3. Castaman G, Matino D. Hemophilia A and B: molecular and clinical similarities and differences. Haematologica. 2019;104(9):1702-1709. 4. Gui T, Reheman A, Ni H, et al. Abnormal hemostasis in a knock-in mouse carrying a variant of factor IX with impaired binding to collagen type IV. J Thromb Haemost. 2009;7(11):1843-1851. 5. Iorio A, Fischer K, Blanchette V, Rangarajan S, Young G, Morfini M; Pharmacokinetic (PK) Expert Working Group of the International Prophylaxis Study Group (the IPSG). Tailoring treatment of haemophilia B: accounting for the distribution and clearance of standard and extended half-life FIX concentrates. Thromb Haemost. 2017;117(6):1023-1030. 6. ALPROLIX. Package insert. Bioverativ Therapeutics Inc; 2023.