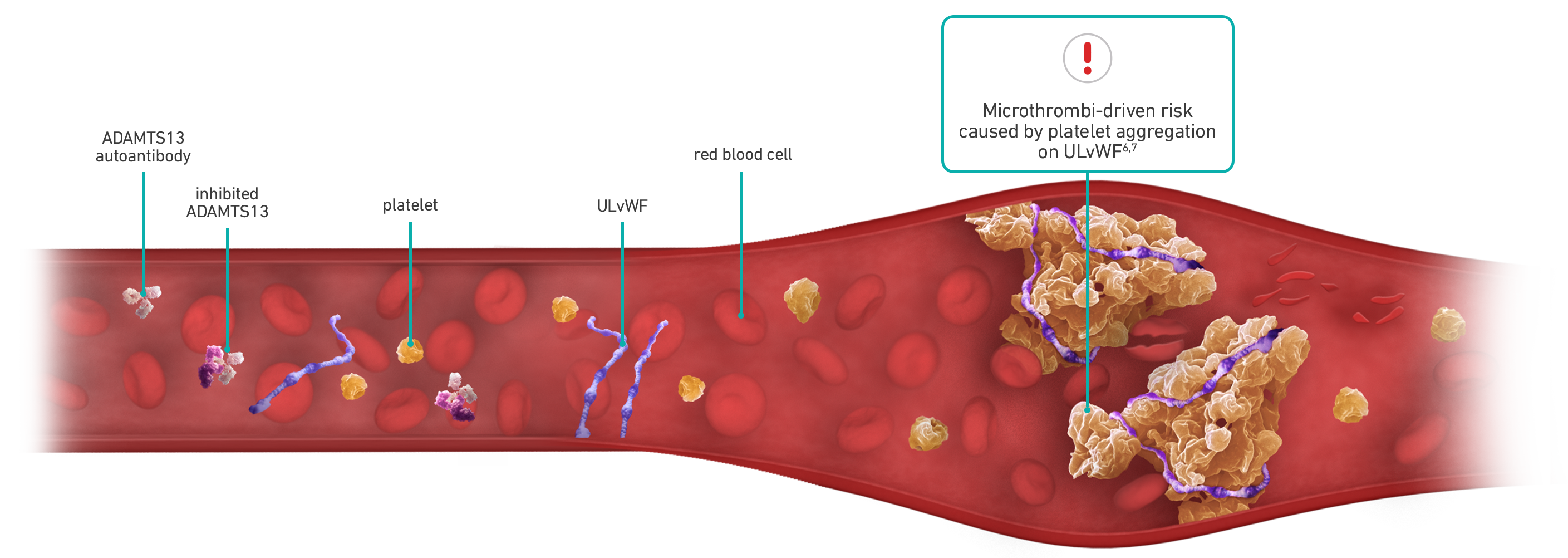
Each aTTP/iTTP episode can be unpredictable with microthrombi-driven risks3-5
CABLIVI is the first and only therapy targeted to block microthrombi in adults with aTTP/iTTP1,2
.png)
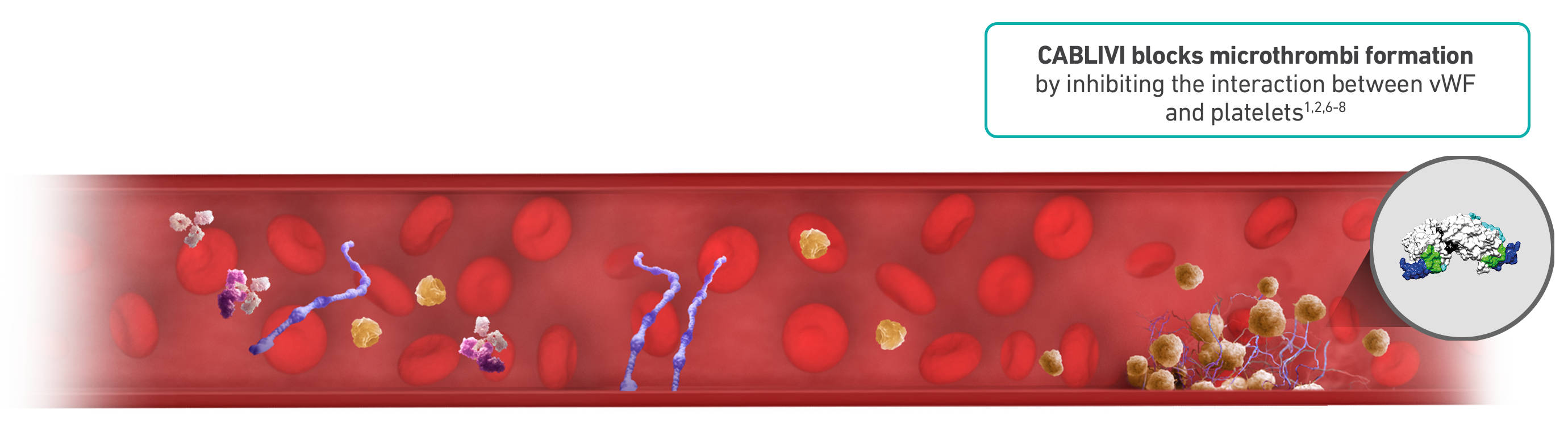
CABLIVI rapidly neutralizes vWF activity, suppressing platelet adhesion from day 1 and throughout treatment9,10
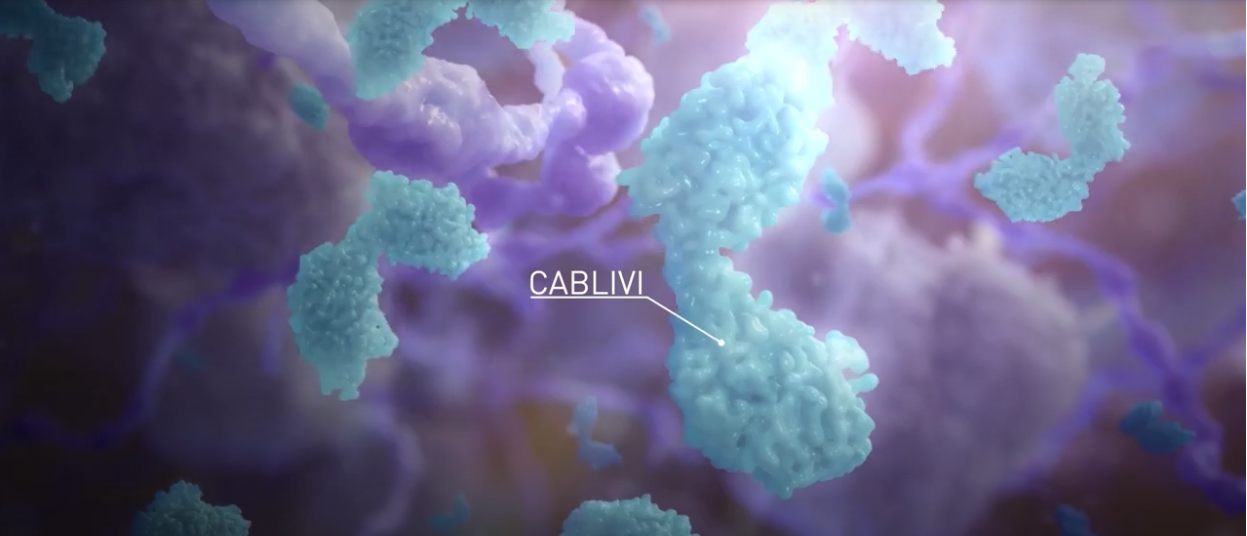
CABLIVI MOA: The first of its kind
Acquired thrombotic thrombocytopenic purpura, or aTTP, is a rare, rapidly progressing, life-threatening autoimmune disease. Left untreated, up to 90% of aTTP patients die.
Historical treatment includes plasma exchange and immunosuppressive therapy, which has improved prognosis. However, despite these treatments, the mortality rate remains high at 8 to 20%. In a large retrospective study, the median time from diagnosis to death was 9 days despite treatment with plasma exchange.
Symptoms of aTTP can be complex and variable. Most common signs include thrombocytopenia, microangiopathic hemolytic anemia, and organ ischemia, resulting from microvascular occlusion. Clinical manifestations of organ ischemia can include strokes, transient ischemic attacks, myocardial infarction, and renal and mesenteric ischemia.
aTTP is caused by the severe deficiency of a von Willebrand factor-cleaving protease, ADAMTS13. This deficiency is due to formation of autoantibodies directed against ADAMTS13. Normally, ADAMTS13 breaks apart ultra-large strings of von Willebrand factor, known as vWF, into smaller pieces. In aTTP patients, however, ADAMTS13 is blocked by autoantibodies, thereby greatly increasing the amount of ultra-large vWF in circulation. Thousands of circulating platelets bind to these ultra-large vWF strings, forming platelet-rich blood clots within small blood vessels throughout the body. These small clots are known as microthrombi.
As of February 2019, CABLIVI was approved by the FDA for aTTP in combination with plasma exchange and immunosuppressive therapy. Plasma exchange removes vWF and autoantibodies and replenishes ADAMTS13. Immunosuppressive therapies are intended to inhibit anti-ADAMTS13 autoantibody production. Neither directly affects the binding of platelets to vWF.
CABLIVI is a monoclonal antibody fragment that was developed to inhibit microthrombi formation in aTTP by binding specifically to the A1 domain of vWF. CABLIVI blocks the interaction between vWF and platelets, thereby reducing both vWF-mediated platelet adhesion and platelet consumption. This inhibits the formation of microthrombi while the underlying autoimmune disease is treated with immunosuppressive therapy.
Who should not start CABLIVI?
- CABLIVI is contraindicated in patients with a previous severe hypersensitivity reaction to caplacizumab-yhdp or to any of its excipients
- Withhold CABLIVI treatment 7 days prior to elective surgery, dental procedures, or other invasive interventions
ADAMTS13=a disintegrin and metalloproteinase with a thrombospondin type 1 motif, 13; aTTP/iTTP=acquired/immune-mediated thrombotic thrombocytopenic purpura; ISTH=International Society on Thrombosis and Haemostasis; MOA=mechanism of action; PEX=plasma exchange; ULvWF=ultra-large von Willebrand factor; vWF=von Willebrand factor.
INDICATION
References: 1. CABLIVI. Prescribing information. Sanofi. 2. Scully M, Cataland SR, Peyvandi F, et al; HERCULES Investigators. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;380(4):335-346. doi:10.1056/NEJMoa1806311 3. Masias C, Wu H, McGookey M, Jay L, Cataland S, Yang S. No major differences in outcomes between the initial and relapse episodes in patients with thrombotic thrombocytopenic purpura: the experience from the Ohio State University Registry. Am J Hematol. 2018;93(3):E73-E75. doi:10.1002/ajh.25002 4. Schieppati F, Russo L, Marchetti M, et al. Low levels of ADAMTS-13 with high anti-ADAMTS-13 antibodies during remission of immune-mediated thrombotic thrombocytopenic purpura highly predict for disease relapse: a multi-institutional study. Am J Hematol. 2020;95(8):953-959. doi:10.1002/ajh.25845 5. Knoebl P, Cataland S, Peyvandi F, et al. Efficacy andsafety of open-label caplacizumab in patients with exacerbations of acquired thrombotic thrombocytopenic purpura in the HERCULES study. J Thromb Haemost. 2019;18(2):479-484. doi:10.1111/jth.14679 6. Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836-2846. doi:10.1182/blood-2016-10-709857 7. Kremer Hovinga JA, Coppo P, Lämmle B, Moake JL, Miyata T,Vanhoorelbeke K. Thrombotic thrombocytopenic purpura. Nat Rev Dis Primers. 2017;3:17020. doi:10.1038/nrdp.2017.20 8. Azoulay E, Bauer PR, Mariotte E, et al; Nine-i Investigators. Expert statement on the ICU management of patients with thrombotic thrombocytopenic purpura. Intensive Care Med. 2019;45(11):1518-1539. doi:10.1007/s00134-019-05736-5 9. Ulrichts H, Silence K, Schoolmeester A, et al. Antithrombotic drug candidate ALX-0081 shows superior preclinical efficacy and safety compared with currently marketed antiplatelet drugs. Blood. 2011;118(3):757-765. doi:10.1182/blood-2010-11-317859 10. Peyvandi F, Scully M, Kremer Hovinga JA, et al; TITAN Investigators. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2016;374(6):511-522. doi:10.1056/NEJMoa1505533
.png)