What is aTTP/iTTP?
Acquired/immune-mediated thrombotic thrombocytopenic purpura (aTTP/iTTP) is a rare, life-threatening, thrombotic microangiopathy manifested as microvascular thrombi and consequent thrombocytopenia, hemolytic anemia, and organ ischemia.2 Thrombotic microangiopathies (TMAs), such as aTTP/iTTP, can be hard to differentiate.6,7
There are 2 types of TTP—aTTP/iTTP and congenital TTP (cTTP), which is caused by mutations in the ADAMTS13 gene. ~95% of TTP cases are aTTP/iTTP.8
Each aTTP/iTTP episode can be unpredictable with microthrombi-driven risks5,9,10
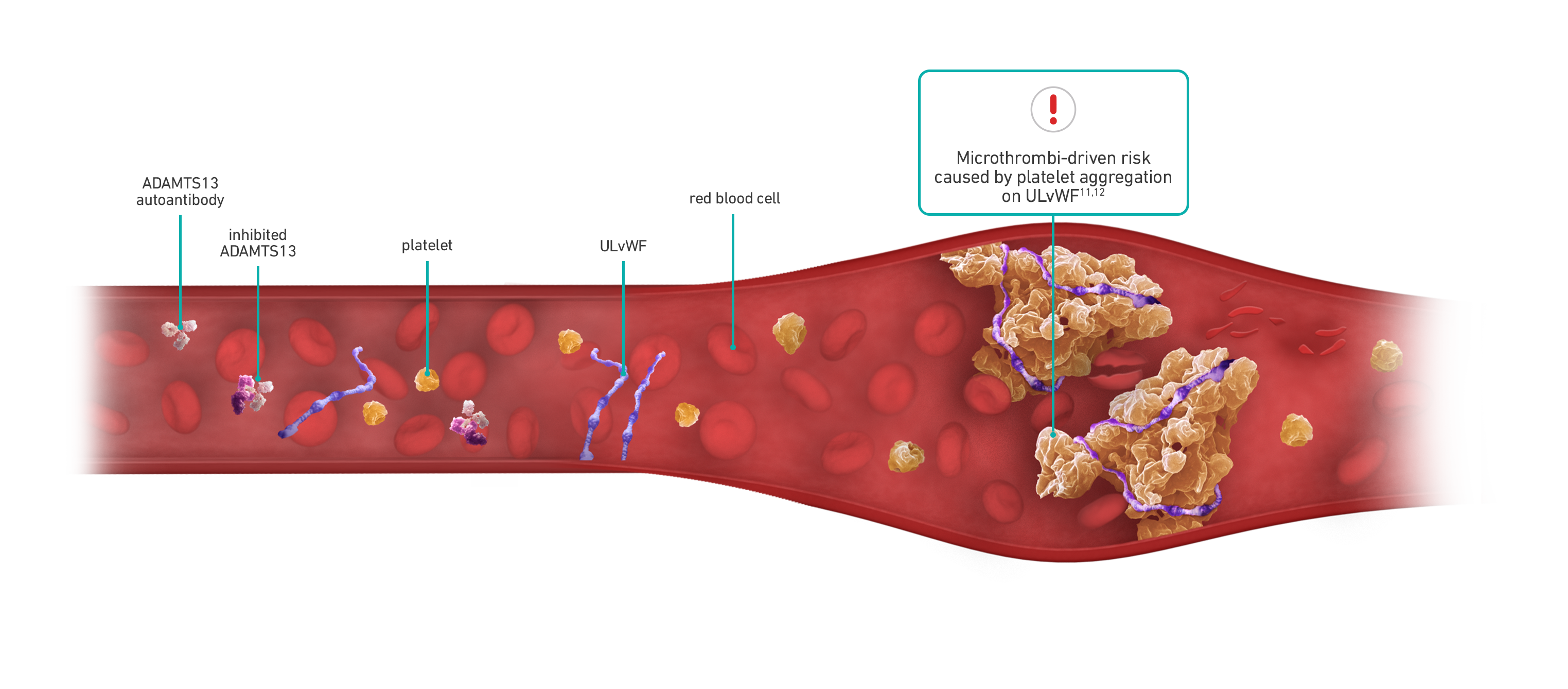
Risk remains for patients with aTTP/iTTP despite treatment with PEX and immunosuppressive therapy13
.png)
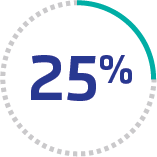
Mortality remains high
25% mortality among patients in the study with an aTTP/iTTP diagnosis, including those treated with PEX and immunosuppressive therapy13*
*Cohort study of 666 patients with aTTP/iTTP across more than 700 hospitals and 7000 clinics in the US from October 2015 to December 2019.
.png)
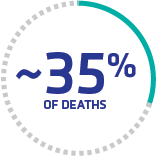
Thromboembolic events caused by ischemia are common
Nearly 35% of in-hospital TTP deaths (613) were related to ischemia, including MI and stroke, despite receiving PEX.4†
†Retrospective claims analysis of hospitalizations with TTP (N=8203).
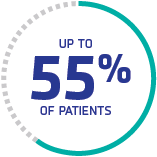
Exacerbation threatens patients
Up to 55% of patients have ≥1 exacerbation within 30 days of stopping PEX.1‡
‡Retrospective review of French Reference Centre for TMA registry (N=388).
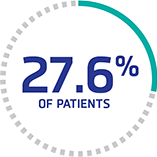
Increased risk of stroke through clinical remission§
Stroke after recovery with PEX occurred in 0% (0/22) of patients with mean normal ADAMTS13 activity (>70%) and in 27.6% (8/29) of patients with ADAMTS13 activity ≤70% (P=0.007).14‖
‖Cohort study of 170 patients with aTTP/iTTP from 1995 to 2018.
§Defined as sustained clinical response either with no PEX and no anti-vWF therapy in the last 30 days or with the attainment of ADAMTS13 remission (partial [between 20% and LLN] or complete [≥LLN]), whichever occurs first.15
ADAMTS13=a disintegrin and metalloproteinase with a thrombospondin type 1 motif, 13; LLN=lower limit of normal; MI=myocardial infarction; PEX=plasma exchange; TTP=thrombotic thrombocytopenic purpura; ULvWF=ultra-large von Willebrand factor; vWF=von Willebrand factor.
INDICATION
References: 1. Grall M, Azoulay E, Galicier L, et al. Thrombotic thrombocytopenic purpura misdiagnosed as autoimmune cytopenia: causes of diagnostic errors and consequence on outcome. Experience of the French Thrombotic Microangiopathies Reference Centre. Am J Hematol. 2017;92(4):381-387. doi:10.1002/ajh.24665 2. Kremer Hovinga JA, Vesely SK, Terrell DR, Lämmle B, George JN. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010;115(8):1500-1511. doi:10.1182/blood-2009-09-243790 3. Peyvandi F, Scully M, Kremer Hovinga JA, et al. Caplacizumab reduces the frequency of major thromboembolic events, exacerbations and death in patients with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2017;15(7):1448-1452. doi:10.1111/jth.13716 4. Goel R, King KE, Takemoto CM, Ness PM, Tobian AAR. Prognostic risk-stratified score for predicting mortality in hospitalized patients with thrombotic thrombocytopenic purpura: nationally representative data from 2007 to 2012. Transfusion. 2016;56(6):1451-1458. doi:10.1111/trf.13586 5. Masias C, Wu H, McGookey M, Jay L, Cataland S, Yang S. No major differences in outcomes between the initial and relapse episodes in patients with thrombotic thrombocytopenic purpura: the experience from the Ohio State University Registry. Am J Hematol. 2018;93(3):E73-E75. doi:10.1002/ajh.25002 6. Coppo P, Veyradier A. Current management and therapeutical perspectives in thrombotic thrombocytopenic purpura. Presse Med. 2012;41(3 pt 2):e163-e176. doi:10.1016/j.lpm.2011.10.024 7. Bommer M, Wölfle-Guter M, Bohl S, Kuchenbauer F. The differential diagnosis and treatment of thrombotic microangiopathies. Dtsch Arztebl Int. 2018;115(19):327-334. doi:10.3238/arztebl.2018.0327 8. Zheng XL, Vesely SK, Cataland SR, et al. ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2020;18(10):2486-2495. doi:10.1111/jth.15006 9. Schieppati F, Russo L, Marchetti M, et al. Low levels of ADAMTS-13 with high anti-ADAMTS-13 antibodies during remission of immune-mediated thrombotic thrombocytopenic purpura highly predict for disease relapse: a multi-institutional study. Am J Hematol. 2020;95(8):953-959. doi:10.1002/ajh.25845 10. Knoebl P, Cataland S, Peyvandi F, et al. Efficacy andsafety of open-label caplacizumab in patients with exacerbations of acquired thrombotic thrombocytopenic purpura in the HERCULES study. J Thromb Haemost. 2019;18(2):479-484. doi:10.1111/jth.14679 11. Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836-2846. doi:10.1182/blood-2016-10-709857 12. Kremer Hovinga JA, Coppo P, Lämmle B, Moake JL, Miyata T, Vanhoorelbeke K. Thrombotic thrombocytopenic purpura. Nat Rev Dis Primers. 2017;3:17020. doi:10.1038/nrdp.2017.20 13. Adeyemi A, Razakariasa F, Chiorean A, De Passos Sousa R. Epidemiology, treatment patterns, clinical outcomes, and disease burden among patients with immune-mediated thrombotic thrombocytopenic purpura in the United States. Res Pract Thromb Haemost. 2022;6(6):e12802. doi:10.1002/rth2.12802 14. Upreti H, Kasmani J, Dane K, et al. Reduced ADAMTS13 activity during TTP remission is associated with stroke in TTP survivors. Blood. 2019;134(13):1037-1045. doi:10.1182/blood.2019001056 15. Cuker A, Cataland SR, Coppo P, et al; International Working Group for Thrombotic Thrombocytopenic Purpura. Redefining outcomes in immune TTP: an international working group consensus report. Blood. 2021;137(14):1855-1861. doi:10.1182/blood.2020009150
.png)