SARCLISA + Pd extended median PFS to ~1 year1
Superior PFS with SARCLISA + Pd vs Pd alone |
At a median follow-up time of 52.4 months, final median OS was 24.6 months in the SARCLISA + Pd arm and 17.7 months in the Pd arm (HR=0.776 [95% CI: 0.594, 1.015]).
The median duration of treatment was 41 weeks with SARCLISA + Pd vs 24 weeks with Pd alone
>40% reduction in the risk of progression or death in patients receiving SARCLISA + Pd
mPFS=median progression-free survival; OS=overall survival; PFS=progression-free survival.
NCCN |
National Comprehensive Cancer Network® (NCCN®) recommends isatuximab-irfc (SARCLISA) in the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Multiple Myeloma as a Category 1 Preferred option in combination with carfilzomib and dexamethasone or with pomalidomide and dexamethasone2:
|
CATEGORY 1 |
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Recommendation for isatuximab-irfc (SARCLISA) in combination with carfilzomib and dexamethasone based on results of interim analysis.
*After 2 prior therapies including lenalidomide and a proteasome inhibitor for isatuximab-irfc in combination with pomalidomide and dexamethasone.
NCCN=National Comprehensive Cancer Network® (NCCN®).
SARCLISA + Pd showed a significant increase in ORR1
ORR: SARCLISA + Pd (95% CI: 0.52, 0.68), Pd (95% CI: 0.28, 0.43). 95% CI estimated using the Clopper-Pearson method.
CR=complete response; ORR=overall response rate; PR=partial response; sCR=stringent complete response; VGPR=very good partial response.
median time to first response with SARCLISA + Pd vs 58 days with Pd alone among responders1
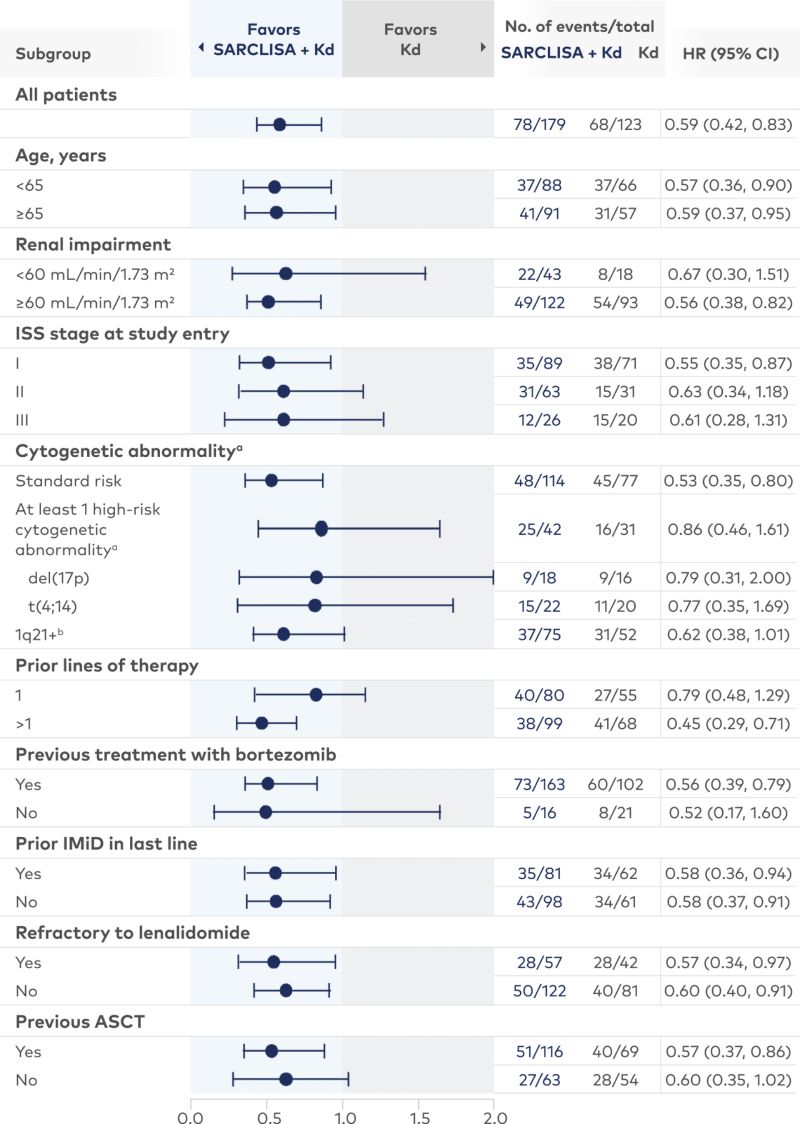
Subgroup data for SARCLISA + Pd3-6
Consistent PFS results were seen across subgroups with SARCLISA + Pd |
.jpg)
aCytogenetic risk information was missing for 18% of patients in the SARCLISA + Pd arm and 26% of patients in the Pd arm. Of the patients who had high-risk chromosomal abnormalities at study entry, del(17p), t(4;14), and t(14;16) were present in 12%, 8%, and 2% of patients, respectively. High-risk cytogenetic status was defined by the presence of ≥1 of del(17p), t(4;14), or t(14;16), and was considered positive if present in ≥30% of plasma cells for t(4;14) and t(14;16), and in ≥50% of plasma cells for del(17p). 1q21+ was considered positive if ≥3 copies of 1q21 were present in ≥30% of analyzed cells.1,3,5
Study limitations
Prespecified subgroup analysis; subgroups were not powered to show differences between treatment arms.
ASCT=autologous stem cell transplant; PI=proteasome inhibitor; R-ISS=Revised International Staging System.
PFS and ORR with SARCLISA + Pd in patients with renal impairment7†
PFS in patients with renal impairment |
Response rates in patients with renal impairment |
Study limitations
Prespecified subgroup analysis; subgroups were not powered to show differences between treatment arms.
†eGFR <60 mL/min/1.73 m2.
eGFR=estimated glomerular filtration rate.
Reduction in the risk of disease progression in patients treated with SARCLISA + Pd vs Pd alone3 |
Study limitations
Prespecified subgroup analysis; subgroups were not powered to show differences between treatment arms.
‡Cytogenetics by central lab; cutoff 50% for del(17p), 30% for t(4;14) and t(14;16).
ICARIA-MM trial: SARCLISA + Pd vs Pd alone1
Evaluated in 307 patients in a phase 3, multicenter, multinational, randomized, open-label study |
Treatment was administered in 28-day cycles until disease progression or unacceptable toxicity.
PRIMARY ENDPOINT: PFS§
Key secondary endpoints: ORR,¶ OS
aSARCLISA 10 mg/kg was administered as an IV infusion weekly in the first cycle and every 2 weeks thereafter.
bPomalidomide 4 mg was taken orally once daily from day 1 to day 21 of each 28-day cycle. Low-dose dexamethasone (orally or IV) 40 mg (20 mg for patients ≥75 years of age) was given on days 1, 8, 15, and 22 for each 28-day cycle.
§PFS results were assessed by an IRC, based on central laboratory data for M-protein, and central radiologic imaging review using the IMWG criteria. Median time to follow-up was 11.6 months.1,4
¶sCR, CR, VGPR, and PR were evaluated by the IRC using the IMWG response criteria.
IMWG=International Myeloma Working Group; IRC=independent response committee; IV=intravenous; M-protein=monoclonal protein.
The ICARIA-MM trial included a broad and diverse patient population1,3-5,8
Baseline characteristics were balanced across both treatment arms |
Patient factors
20% | Elderly (≥75 years) |
36% | Impaired renal function‖ |
20% | High cytogenetic risk |
Treatment history
93% | Refractory to lenalidomide |
73% | Refractory to IMiD + Pl |
56% | Prior ASCT |
| Baseline characteristics | SARCLISA + Pd (n=154) | Pd (n=153) |
| Age, years | ||
| <65 | 35% | 46% |
| 65-74 | 44% | 35% |
| ≥75 | 21% | 19% |
| R-ISS stage at study entry | ||
| I | 25% | 20% |
| II | 64% | 64% |
| III | 10% | 16% |
| Cytogenetic riska | ||
| High | 16% | 24% |
| Standard | 67% | 51% |
| Missing | 18% | 26% |
| 1q21+ | 49% | 34% |
| Renal function | ||
| <60 mL/min/1.73 m2 | 39% | 34% |
| History of COPD or asthma at study entry | ||
| Yes | 10% | 11% |
| ECOG PS | ||
| 0 or 1 | 90% | 90% |
| 2 | 10% | 10% |
| Prior lines of therapy | ||
| Median (range) | 3 (2-11) | 3 (2-10) |
| Patient refractory to | ||
| PI (bortezomib) | 77% (62%) | 75% (58%) |
| IMiD (lenalidomide) | 96% (94%) | 94% (92%) |
| IMiD + PI | 73% | 72% |
| Last regimen | 97% | 99% |
| Prior ASCT | ||
| Yes | 54% | 59% |
aOf the patients who had high-risk chromosomal abnormalities at study entry, del(17p), t(4;14), and t(14;16) were present in 12.1%, 8.5%, and 1.6% of patients, respectively. The 1q21+ subgroup was evaluated in a retrospective analysis using the remaining CD138+ plasma cells collected for cytogenetic risk evaluation and was considered positive if ≥3 copies of 1q21 were present in ≥30% of analyzed cells.4,5
‖Information on race could not be collected in some countries; hence, not all values were available. The percentage was calculated using N=287 and included 55/142 patients in the SARCLISA + Pd arm and 49/145 patients in the Pd arm.3
COPD=chronic obstructive pulmonary disease; ECOG PS=Eastern Cooperative Oncology Group performance status; IMiD=immunomodulatory drug.
Important Safety Information
References: 1. SARCLISA. Prescribing information. sanofi-aventis U.S. LLC; 2024. 2. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Multiple Myeloma V.1.2025. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed September 24, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org. 3. Attal M, Richardson PG, Rajkumar SV, et al; ICARIA-MM study group. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394(10214):2096-2107. 4. Data on file. sanofi-aventis U.S. LLC. 5. Harrison SJ, Perrot A, Alegre A, et al. Subgroup analysis of ICARIA-MM study in relapsed/refractory multiple myeloma patients with high-risk cytogenetics. Br J Haematol. 2021;194(1):120-131. 6. Supplement to: Harrison SJ, Perrot A, Alegre A, et al. Subgroup analysis of ICARIA-MM study in relapsed/refractory multiple myeloma patients with high-risk cytogenetics. Br J Haematol. 2021;194(1):120-131. 7. Dimopoulos MA, Leleu X, Moreau P, et al. Isatuximab plus pomalidomide and dexamethasone in relapsed/refractory multiple myeloma patients with renal impairment: ICARIA-MM subgroup analysis. Leukemia. 2020;35(2):562-572. doi:10.1038/s41375-020-0868-z 8. Hájek R, Jarkovsky J, Maisnar V, et al. Real-world outcomes of multiple myeloma: retrospective analysis of the Czech Registry of Monoclonal Gammopathies. Clin Lymphoma Myeloma Leuk. 2018;18(6):e219-e240.
